Chronic obstructive pulmonary disease and obstructive sleep apnoea—the overlap syndrome
Introduction
Chronic obstructive pulmonary disease (COPD) and obstructive sleep apnoea syndrome (OSAS) are highly prevalent disorders (1-3), so the possibility of both occurring together in the same patient is relatively high by chance alone. Current estimates for the prevalence of COPD are in the region of 10% (1,4) and the prevalence of OSAS is at least 10% (3,5). The co-existence of both disorders, termed the overlap syndrome, carries additional prognostic implications relating to worsening respiratory failure, cardiovascular and other co-morbidities, and ultimately survival (6). The present review addresses epidemiology, pathophysiology, clinical assessment, management, and implications for co-morbidities of the overlap syndrome.
Epidemiology
The most prevalent chronic respiratory disorders are COPD, asthma and OSA. COPD prevalence is related to the prevalence of tobacco smoking but reports indicate that 10% of the general population around the world have moderate to severe COPD [forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <0.7 plus FEV1 <80% of predicted] (4). The prevalence of OSA is equally high with over 25% of adult males having an apnoea hyponea index (AHI) >5 and up to 15% having AHI>5 with associated excessive daytime sleepiness (EDS) (3,7,8). Prevalence figures for both disorders are influenced by definitions such as the presence of symptoms in addition to objective abnormalities. Furthermore, the prevalence of OSA has been increasing over recent decades, most likely as a consequence of the rising prevalence of obesity (3). Both disorders have a high global prevalence and affect all socio-economic groups.
Based on prevalence figures for each disorder alone, it can be estimated that at least one percent of the general population will have at least some degree of both conditions together, but several studies have explored the possibility of a higher prevalence than would be expected from simple coincidence. A report based on data from the Sleep Heart Health Study (9) found a relatively low rate of OSA in patients with lower airway obstruction, but this was a consequence of lower body mass index (BMI), as AHI was similar when values were stratified for BMI. Similar findings were reported by the MONICA (Multinational Monitoring of Trends and Determinants in Cardiovascular Disease) II project, sponsored by the World Health Organisation (10). In this study, AHI >5 with sleepiness was found in 11.3% of subjects and 10.7% had FEV1/FVC ratio <70%, but lower airway obstruction did not predispose to OSAS, or vice versa.
More recent studies suggest that the prevalence of each disorder together may be higher than that predicted by chance occurrence alone. An Israeli study reported that COPD was more prevalent in patients with OSA with a reported odds ratio (OR) for COPD in males of 1.8 and in females of 4.3 compared to matched controls (11). Furthermore, the recent report of Soler and co-authors (12) found a high prevalence of OSA in patients with severe COPD, particularly when overweight. Thus, this question remains uncertain and warrants further study.
Pathophysiology
Sleep has a number of adverse effects on breathing that include negative effects on respiratory control, respiratory muscle function, and lung mechanics (13). These effects produce negligible adverse consequences in normal subjects but may result in profound disturbances of gas exchange in patients with COPD. These adverse effects of sleep on gas exchange have been recognised for many years and patients with COPD may experience profound oxygen desaturation, particularly during rapid-eye-movement (REM) sleep, in addition to carbon dioxide retention, and the oxygen desaturation encountered during sleep may exceed that during maximum exercise (14). This hypoxaemia predisposes to arrhythmias (15), pulmonary hypertension (16) and nocturnal death, particularly during acute exacerbations (17). Furthermore, patients with COPD experience poor sleep quality with diminished amounts of slow-wave and REM sleep (18,19).
An important factor that contributes to disordered breathing during sleep in patients with COPD is the diminution of skeletal muscle function, especially during REM sleep, which particularly affects the accessory muscles of respiration such as the intercostal muscles (20). These effects are not peculiar to patients with OSA, and a number of additional factors can be considered that may influence the relationship between COPD and OSA, which are summarised in Table 1. Factors that may promote the development of OSA include rostral fluid shift during sleep when supine (21), which is particularly relevant to patients with cor pulmonale where peripheral oedema is a common finding. Additional factors include cigarette smoking, which contributes to upper airway inflammation and oedema, and certain medications such as corticosteroids which contribute to central fat deposition (6). On the other hand, several factors relevant to COPD may protect against the development of OSA, including low BMI, diminished REM sleep, and certain medications used in patients with COPD such as theophylline (22). A recent report identified BMI and pack years smoking as major predictors of OSA among patients with COPD (23), and awake hypoxaemia, hypercapnia, and pulmonary hypertension are more common in patients with the overlap syndrome than in those with either condition alone (24).
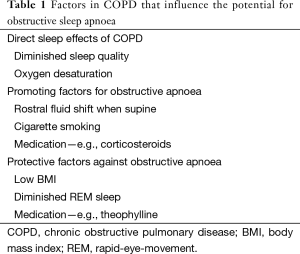
Full table
Clinical assessment
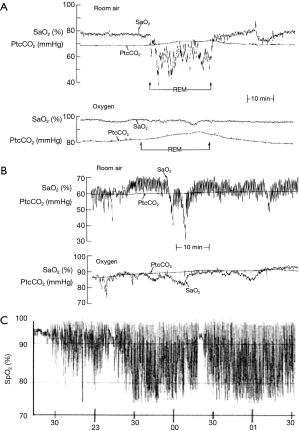
While the gold standard investigation for sleep disordered breathing is overnight polysomnography (25), this investigation is not practical for the large numbers of patients susceptible to the overlap syndrome. Since nocturnal oxygen desaturation is more pronounced in patients with the overlap syndrome, overnight oximetry represents a simple screening tool for these patients. The desaturation characteristics may also provide insight into the pathophysiology of sleep-disordered breathing, as patients OSA typically demonstrate a profile of intermittent desaturation whereas patients with hypoventilation demonstrate a more sustained pattern of oxygen desaturation during sleep. An example of nocturnal saturation profiles in the different disorders is presented in Figure 1. Sleep apnoea per se is better demonstrated by ambulatory cardiorespiratory polygraphy (26), which represents a suitable specific investigation for this possibility.
Patients with COPD typically report poor sleep quality and daytime fatigue, whereas patients with OSA typically report snoring, broken and unrefreshing sleep, in addition to EDS. Thus, there is clear overlap in symptom profile between the two disorders which reinforces the importance of considering the possibility of overlap when patients present for assessment.
Management
Oxygen therapy remains the principal management of COPD patients with associated hypoxaemia. There are documented survival advantages (27) and the risk of carbon dioxide retention with controlled oxygen therapy is relatively low (28,29). Optimising lung function will also benefit oxygenation, and most available bronchodilators have demonstrated efficacy in this regard (30-32).
The role of non-invasive pressure support ventilation (NIV) in the management of COPD patients with nocturnal sleep disordered breathing is not clear-cut. While NIV has established efficacy in the management of respiratory failure during acute exacerbations of COPD (33), the role of nocturnal NIV in stable hypercapnic COPD patients remains uncertain. A Cochrane review on the subject, published in 2013, concluded that there was no evidence for a consistent clinical or statistically significant beneficial effect on gas exchange, exercise tolerance, health-related quality of life (HRQoL), lung function, respiratory muscle strength or sleep efficiency; and furthermore, a meta-analysis of two long-term studies did not show significant improvements in blood gases, HRQoL or lung function after 12 months of NIV (34). Another more recent meta-analysis from 2014 (35) agreed with the Cochrane findings but reported that higher inspiratory positive airway pressure (IPAP) levels, better compliance and higher baseline arterial carbon dioxide tensions (PaCO2) appeared to improve PaCO2.
Building on this recent meta-analysis, the recent report of Köhnlein and co-authors indicated that NIV targeted to markedly reduce hypercapnia (>20%) compared to best usual care in patients with advanced stable hypercapnic COPD resulted in lower 1-year all-cause mortality and improved quality of life scores (36).
There are few studies that have specifically addressed the role of NIV in patients with the overlap syndrome. Marin and co-workers have provided probably the best data on this topic in a long-term study comparing the outcomes in three groups with over 200 patients each: overlap patients treated with NIV, overlap patients not treated with NIV, and compared with COPD patients without OSA (37). Over a 12-year follow-up period, the survival was significantly better among overlap patients treated with NIV, and not significantly different than patients with COPD alone (Figure 2).

Co-morbidities
COPD (38) and OSA (39) have separately been identified as independently associated with several co-morbidities, most notably cardiovascular. Thus, one could anticipate that co-morbidities would be more prevalent in patients with the overlap syndrome than with either disease alone. However, there is little published evidence on the relative prevalence of cardiovascular or other co-morbidities in patients with the overlap syndrome compared to patients with COPD or OSA alone.
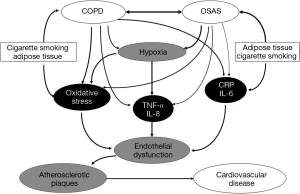
A variety of molecular pathways have been identified as potential contributors to co-morbidity, including systemic inflammation mediated by C-reactive protein (CRP) and by the downstream products of nuclear transcription factor-kappa B (NF-kB) activation such as tumour necrosis factor-alpha (TNF-α) and interleukin-8 (IL-8) (6). These inflammatory mechanisms have been identified as important mechanisms in cardiovascular disease, and each has been associated with both COPD (40) and OSA (41-43). Whether the overlap of each disorder amplifies these responses is unclear, but the additional burden of hypoxia in the overlap syndrome could be an important mechanism in this regard, particularly in the context of TNF-α (44). Additional shared mechanisms that likely play a role in the development of cardiovascular co-morbidity include oxidative stress (45,46) and increased neutrophil productions and/or dysfunction (40,47). This topic represents an important area for future research and it cannot be assumed that inflammatory processes will necessarily be amplified, as the mechanisms involved may not be synergistic. For example, TNF-α elevation in COPD is associated with muscle wasting (48), likely relating to TNF-α accumulation in muscle tissue, which could be less relevant in patients with the overlap syndrome. However, these aspects remain unexplored. A potential mechanistic interaction between COPD and OSA in the development of cardiovascular co-morbidity is given in Figure 3.
Conclusions
COPD and OSA are both highly prevalent disorders but whether each disorder predisposes to a higher incidence of the other is unclear. While sleep can produce clinically significant detrimental effects on sleep quality and gas exchange in patients with COPD, many clinicians ignore this aspect, despite the fact that appropriate management may produce considerable benefits to gas exchange and quality of life. The role of NIV is well established in acute exacerbations of COPD but less so in the chronic setting. However, patients with the overlap syndrome clearly benefit from continuous positive airway pressure (CPAP), particularly in long-term survival. Overlapping co-morbidities are prevalent in each disorder, but whether this association is amplified in patients with the overlap syndrome remains unclear. Thus, there remains a major opportunity for further research in this area, which appears particularly important given the relatively high prevalence of the overlap syndrome in the general population.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932-46. [PubMed]
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765-73. [PubMed]
- Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006-14. [PubMed]
- Celli BR, Halbert RJ, Isonaka S, et al. Population impact of different definitions of airway obstruction. Eur Respir J 2003;22:268-73. [PubMed]
- Lévy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnoea syndrome. Nature Reviews Disease Primers 2015;1:1-20. [PubMed]
- McNicholas WT. Chronic obstructive pulmonary disease and obstructive sleep apnea: overlaps in pathophysiology, systemic inflammation, and cardiovascular disease. Am J Respir Crit Care Med 2009;180:692-700. [PubMed]
- Tufik S, Santos-Silva R, Taddei JA, et al. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med 2010;11:441-6. [PubMed]
- Durán J, Esnaola S, Rubio R, et al. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med 2001;163:685-9. [PubMed]
- Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med 2003;167:7-14. [PubMed]
- Bednarek M, Plywaczewski R, Jonczak L, et al. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respiration 2005;72:142-9. [PubMed]
- Greenberg-Dotan S, Reuveni H, Tal A, et al. Increased prevalence of obstructive lung disease in patients with obstructive sleep apnea. Sleep Breath 2014;18:69-75. [PubMed]
- Soler X, Gaio E, Powell FL, et al. High Prevalence of Obstructive Sleep Apnea in Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc 2015;12:1219-25. [PubMed]
- McNicholas WT. Impact of sleep in COPD. Chest 2000;117:48S-53S. [PubMed]
- Mulloy E, McNicholas WT. Ventilation and gas exchange during sleep and exercise in severe COPD. Chest 1996;109:387-94. [PubMed]
- Tirlapur VG, Mir MA. Nocturnal hypoxemia and associated electrocardiographic changes in patients with chronic obstructive airways disease. N Engl J Med 1982;306:125-30. [PubMed]
- Fletcher EC, Luckett RA, Miller T, et al. Pulmonary vascular hemodynamics in chronic lung disease patients with and without oxyhemoglobin desaturation during sleep. Chest 1989;95:757-64. [PubMed]
- McNicholas WT, Fitzgerald MX. Nocturnal deaths among patients with chronic bronchitis and emphysema. Br Med J (Clin Res Ed) 1984;289:878. [PubMed]
- Agusti A, Hedner J, Marin JM, et al. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev 2011;20:183-94. [PubMed]
- McSharry DG, Ryan S, Calverley P, et al. Sleep quality in chronic obstructive pulmonary disease. Respirology 2012;17:1119-24. [PubMed]
- Johnson MW, Remmers JE. Accessory muscle activity during sleep in chronic obstructive pulmonary disease. J Appl Physiol Respir Environ Exerc Physiol 1984;57:1011-7. [PubMed]
- White LH, Bradley TD. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol 2013;591:1179-93. [PubMed]
- Mulloy E, McNicholas WT. Theophylline in obstructive sleep apnea. A double-blind evaluation. Chest 1992;101:753-7. [PubMed]
- Steveling EH, Clarenbach CF, Miedinger D, et al. Predictors of the overlap syndrome and its association with comorbidities in patients with chronic obstructive pulmonary disease. Respiration 2014;88:451-7. [PubMed]
- Chaouat A, Weitzenblum E, Kessler R, et al. Sleep-related O2 desaturation and daytime pulmonary haemodynamics in COPD patients with mild hypoxaemia. Eur Respir J 1997;10:1730-5. [PubMed]
- McNicholas WT. Diagnosis of obstructive sleep apnea in adults. Proc Am Thorac Soc 2008;5:154-60. [PubMed]
- Kuna ST, Badr MS, Kimoff RJ, et al. An official ATS/AASM/ACCP/ERS workshop report: Research priorities in ambulatory management of adults with obstructive sleep apnea. Proc Am Thorac Soc 2011;8:1-16. [PubMed]
- Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet 1981;1:681-6. [PubMed]
- Goldstein RS, Ramcharan V, Bowes G, et al. Effect of supplemental nocturnal oxygen on gas exchange in patients with severe obstructive lung disease. N Engl J Med 1984;310:425-9. [PubMed]
- Moloney ED, Kiely JL, McNicholas WT. Controlled oxygen therapy and carbon dioxide retention during exacerbations of chronic obstructive pulmonary disease. Lancet 2001;357:526-8. [PubMed]
- McNicholas WT, Calverley PM, Lee A, et al. Long-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPD. Eur Respir J 2004;23:825-31. [PubMed]
- Mulloy E, McNicholas WT. Theophylline improves gas exchange during rest, exercise, and sleep in severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1993;148:1030-6. [PubMed]
- Ryan S, Doherty LS, Rock C, et al. Effects of salmeterol on sleeping oxygen saturation in chronic obstructive pulmonary disease. Respiration 2010;79:475-81. [PubMed]
- Brochard L, Isabey D, Piquet J, et al. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med 1990;323:1523-30. [PubMed]
- Struik FM, Lacasse Y, Goldstein R, et al. Nocturnal non-invasive positive pressure ventilation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013;6:CD002878. [PubMed]
- Struik FM, Lacasse Y, Goldstein RS, et al. Nocturnal noninvasive positive pressure ventilation in stable COPD: a systematic review and individual patient data meta-analysis. Respir Med 2014;108:329-37. [PubMed]
- Köhnlein T, Windisch W, Köhler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med 2014;2:698-705. [PubMed]
- Marin JM, Soriano JB, Carrizo SJ, et al. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med 2010;182:325-31. [PubMed]
- Macnee W, Maclay J, McAllister D. Cardiovascular injury and repair in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;5:824-33. [PubMed]
- McNicholas WT, Bonsigore MR. Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 2007;29:156-78. [PubMed]
- Gan WQ, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004;59:574-80. [PubMed]
- Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 2005;112:2660-7. [PubMed]
- Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2006;174:824-30. [PubMed]
- Ryan S, McNicholas WT. Intermittent hypoxia and activation of inflammatory molecular pathways in OSAS. Arch Physiol Biochem 2008;114:261-6. [PubMed]
- Takabatake N, Nakamura H, Abe S, et al. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;161:1179-84. [PubMed]
- Lavie L. Obstructive sleep apnoea syndrome--an oxidative stress disorder. Sleep Med Rev 2003;7:35-51. [PubMed]
- MacNee W. Oxidants/antioxidants and COPD. Chest 2000;117:303S-17S. [PubMed]
- Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med 2002;165:934-9. [PubMed]
- Eid AA, Ionescu AA, Nixon LS, et al. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1414-8. [PubMed]


