miR-1290 is a potential prognostic biomarker in non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, due to its high incidence, malignant behavior, and lack of effective treatment strategy. The prognosis for lung cancer is very poor despite recent advances in diagnosis and therapies, and the five-year survival rate of lung cancer patients is less than 15% (1,2). Approximately 85% of all lung cancer cases are categorized as non-small cell lung cancer (NSCLC), and most patients present with advanced disease at the time of diagnosis further contributing to dismal morbidity and mortality rates (3). Consequently, to provide better treatment strategies, there is an urgent need to identify new biomarkers and therapeutic targets for lung cancer.
MicroRNAs (miRNAs) are a newly-discovered class of small non-coding RNAs (19-22 nucleotides), and have been identified as important factors in cancer tumorigenesis and progression (4,5). miRNAs expression profiles not only allow distinguishing malignant and non-malignant tissue, but also distinguishing different tumor entities and stages (6). In addition, specific miRNAs are useful to distinguish cancer patients and healthy controls (7). Patients with breast cancer have increased levels of miR-195 (8), and miR-26a has been evaluated in prostate cancer (9). Furthermore, miR-29 is a prognostic indicator for colon cancer (10). Emerging evidence supports a role for miRNAs in multiple cancer development and progression, including lung cancer (11,12).
miR-1290 was initially discovered in human embryonic stem cells, which was encoded in the first intron of the aldehyde dehydrogenase 4 family, member A1 (ALDH4A1) gene (13). Yelamanchili et al. (14) identified that miR-1290 could regulate the human neuronal differentiation process by acting on critical cell cycle proteins. Li et al. (15) found that miR-1290 was overexpressed in primary pancreatic cancer tissues, and patients with higher serum miR-1290 levels had worse prognosis. However, the role of miR-1290 in lung cancer is still unclear.
In the present study, miR-1290 expression levels in human NSCLC tissues and serum was examined by specific TaqMan qRT-PCR. The correlation between miR-1290 and clinical characteristics and prognosis was subsequently analyzed.
Materials and methods
Tissues specimens
The study was approved by the Ethical Committee of The First Affiliated Hospital of Nanjing Medical University (Nanjing, China), and informed consent was obtained from all patients. NSCLC tissue samples and matched non-tumor adjacent tissues (NATs) were obtained from patients who underwent surgical resection at the Thoracic Surgery Department of First Affiliated Hospital of Nanjing Medical University, between March 2010 and December 2011 and were diagnosed with NSCLC based on histopathological evaluation. All tissues were immediately snap-frozen in liquid nitrogen and stored at −80 °C until use. In addition, the patients with any other tumor were excluded from the study. A total of 33 pairs of NSCLC tissues were examined in the study. According to the criteria of the Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC), seventh edition, 8, 13, and 12 patients exhibited stage I, II, and IIIa cancer, respectively. None of the subjects had received any therapeutic procedures prior to this study, including surgery, chemotherapy, and radiotherapy.
Serum samples
To further determine the role of miR-1290 in NSCLC, serum samples were collected. From March 2009 to December 2013, we collected serum samples from patients who visited the Thoracic Surgery Department and the Oncology Department of The First Affiliated Hospital of Nanjing Medical University (Nanjing, China). Patients with recent history of any cancer other than lung cancer were excluded. We included patients with NSCLC who had not received any therapeutic procedures before collection of serum samples. A total of 73 patients were involved in this study, and 17 Is, 14 IIs, 4 IIIas, 16 IIIbs, and 22 IVs were identified, according to the criteria of the UICC/AJCC, seventh edition. Furthermore, serum from 19 patients with various benign lung disease and 34 cases of healthy controls were also obtained. Serum samples were extracted from whole blood after centrifugation (2,800 g, 10 min) and stored at −80 °C until further processing. The prognosis was evaluated in all NSCLC patients with serum samples in April 2015. Overall survival was defined as the time from cancer onset until death or by censoring at the last follow-up date.
Isolation of total RNA and quantitative RT-PCR
Total RNAs of tissues and serum samples were extracted with Qiagen miRNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol, and then both miRNAs and mRNA were reverse transcribed to cDNA. TaqMan miRNA assays (Applied Biosystems, Foster City, USA) with specific RT primers and probes were used to quantify the expression of mature has-miR-1290 (5'-UGGAUUUUUGGAUCAGGGA-3'). cDNA was generated from 500 ng of total RNA using PrimeScript™ RT Master Mix Perfect Real Time (TaKaRa, Dalian, China). Quantitative real-time PCR for ALDH4A1 was then performed with SYBR® Premix DimerEraser (TaKaRa). U6 was used for miRNA template normalization and β-actin for mRNA template normalization. The relative expression level of target RNAs was calculated by 2−∆∆Ct method, in which ∆∆Ct = ∆Ct (target − reference) (in tumor samples) − ∆Ct (target − reference) (in NATs) (16). As for serum samples, 100 fmol/ml of synthesized cel-miR-39 (Qiagen, Hilden, Germany) was added to equal volume of serum to serve as normalizer before RNA extraction. The relative levels of miR-1290 in serum were expressed as 2−∆Ct method, in which ∆Ct = CtmiR-1290 − Ctcel-miR-39.
The primers sequences were as follows:
ALDH4A1 forward, 5'-CCATCTCGCCCTTTAACTTCAC-3'; ALDH4A1 reverse, 5'-ACTGGGCTTCCATAGGACCA-3';
β-actin forward, 5'-TGGCCCCAGCACAATGAA-3'; β-actin reverse, 5'-CTAAGTCATAGTCCGCCTAGAAGCA-3'.
RT-PCR was performed by using the ABI 7500 fast real-time PCR system (Applied Biosystems, Foster City, USA). All samples were performed in triplicate and independently repeated three times.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics Version 16 (SPSS Inc, Chicago, IL, USA) and GraphPad Prism v5.0 (Graphpad Software Inc.). The Wilcoxon test was used to compare miR-1290 expression in paired tumor tissue samples and NATs. The Mann-Whitney U test and Kruskal-Wallis test were used to perform statistical analysis of serum miR-1290 levels between unpaired groups and multiple comparison groups, respectively. The Pearson’s chi-squared test and Fisher’s exact test were used to evaluate the association between serum or tissue miRNA levels and clinicopathological parameters. The Spearman correlation test was used to examine correlation between the expression of ALDH4A1 mRNA and miR-1290 in tumor tissues. In addition, survival curves were constructed with the Kaplan-Meier method and compared using log-rank test. Cox proportional hazards regression analysis was used for univariate and multivariate analyses of prognostic values. P value of two-sided less than 0.05 was considered statistically significant.
Results
miRNA-1290 and ALDH4A1 mRNA expression in NSCLC tissues
To analyze the expression of miR-1290 in patients with NSCLC, we measured the levels of miR-1290 in 33 pairs of NSCLC tissues and the NATs. Significantly higher miR-1290 was detected in tumor tissues compared with the NATs (P<0.001) (Figure 1A). Data are presented as log2 of fold-change (cancer/normal) and defined as “>1”as overexpression, “<−1” as underexpression, and the remaining were unchanged (17). The results showed that 18 cases (54.5%) had significantly increased levels of miR-1290 in NSCLC tissues compared with their NATs. There were 15 cases (45.5%) in whom the expression of miR-1290 was slightly changed (Figure 1B). miR-1290 is encoded in the first intron of ALDH4A1. Thus, we also examined mRNA expression of ALDH4A1 in NSCLC tissues. We did not find any significant difference in ALDH4A1 expression level between paired tumor tissues and NATs (P=0.124; Figure 1C). Furthermore, the correlation between ALDH4A1 mRNA and miR-1290 in tissues was analyzed with the spearman correlation test. However no significant correlation was observed (r=0.334; P=0.057; Figure 1D).
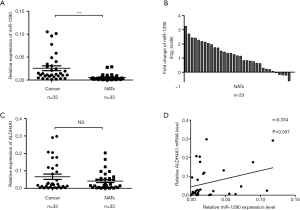
Association between clinicopathological features and miR-1290 expression levels in NSCLC tissues
The association between tissues miR-1290 expression levels and clinicopathological parameters were summarized in Table 1. For the NSCLC samples, high expression of miR-1290 was significantly more frequently observed in stage IIIa samples than in stage I and II samples (P=0.004). Moreover, we noted significant correlation between expression levels of miR-1290 and lymph node metastasis (P=0.013). However, statistical analysis revealed no significant correlation between expression of miR-1290 and age, gender, smoking history, tumor size, and histology type (P=0.401, 0.228, 0.169, 0.868, and 0.808, respectively; Table 1).
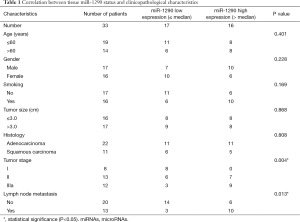
Full table
Serum miR-1290 expression levels in NSCLC
We further detected miR-1290 expression levels in serum. A total of 126 serum samples, including those from patients with NSCLC (n=73), patients with benign lung disease (n=19), and healthy controls (n=34) were examined. The expression levels of serum miR-1290 were significantly higher in the NSCLC group than in the benign lung disease group (P=0.028) and healthy control group (P<0.001; Figure 2). Furthermore, when all NSCLC serum samples were segregated based upon TNM stage, the gradual increase in serum miR-1290 expression levels was clearly discernible. Serum levels of miR-1290 expression associated with TNM stage (stage I vs. IIIb: P=0.007; stage I vs. IV: P<0.001) (Figure 2).
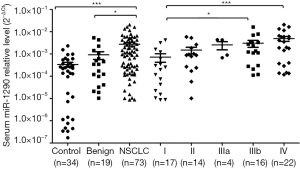
Correlation of serum miR-1290 with clinicopathological factors
The correlation of serum miR-1290 expression levels with clinicopathological factors of NSCLC patients was statistically analyzed in Table 2. High level of serum miR-1290 expression was significantly correlated with high TNM stage (P=0.022) and lymph node metastasis (P=0.024). However, there were no obvious changes between serum miR-1290 expression levels and other factors including age, gender, smoking history, and histological type (P=0.816, 0.607, 0.351, and 0.794, respectively).
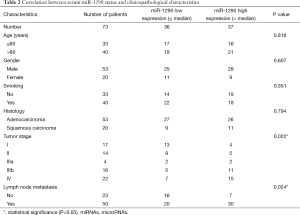
Full table
Correlation between serum miR-1290 level and patients’ survival
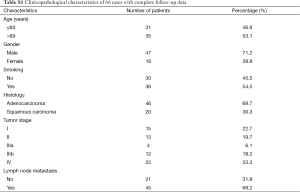
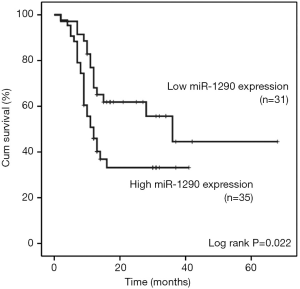
We further evaluated the association between serum miR-1290 levels and overall survival of NSCLC patients. Overall survival curves of NSCLC patients were estimated using the Kaplan-Meier method and compared with log-rank test. Complete follow-up data were available for 66 cases, which were categorized as low miR-1290 expression and high miR-1290 expression (based on the median expression). The clinicopathological characteristics of these 66 cases were shown in Table S1. Assessment of survival in NSCLC patients revealed that higher expression of miR-1290 was correlated with adverse survival of patients. As of April 2015, 39 patients (53.4%) had died during the follow-up period. Although the mortality rate was 53.4% for patients, patients with low miR-1290 expression had a longer survival time (months) compared with the patients with high miR-1290 expression (13.14±1.65 vs. 8.8±1.04, P=0.037). The median observation time for overall survival was 12 months (range, 2-68 months). The survival rate for patients with low miR-1290 expression was 51.7% compared with only 31.5% for patients with high miR-1290 expression. The median survival time of patients with high-level expression of miR-1290 was only 12 months, whereas the median survival time of those with low expression levels of miR-1290 was 36 months (log-rank test: P=0.022 ; Figure 3).
Full table
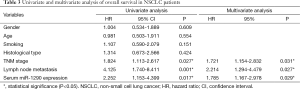
Univariate and multivariate Cox analysis for prognosis of patients with NSCLC
Next, we performed a univariate Cox proportional hazards regression analysis to determine the influence of serum miR-1290 levels and clinicopathological characteristics (gender, age, smoking history, histology type, TNM stage, and lymph node metastasis) on patient survival. The results showed that TNM stage, lymph node metastasis, and serum miR-1290 expression levels were significantly correlated with overall survival (P=0.027, 0.001, and 0.017, respectively). Multivariate analysis was used to assess the parameters that significantly correlated with survival in the univariate analysis. Results of the multivariate analysis indicated that TNM stage and lymph node metastasis status [hazard ratio (HR) =1.721; 95% confidence interval (CI), 1.154-2.832; P=0.031 and HR =2.214; 95% CI, 1.294-4.479; P=0.027, respectively] and serum miR-1290 expression (HR =1.785; 95% CI, 1.167-2.978; P=0.029) were the independent prognostic factors for overall survival (Table 3).
Full table
Discussion
miRNA functional studies have suggested deregulation of miRNAs as a factor in the initiation and progression of cancer, though the mechanisms are still largely unknown (18-20). During the past few years, the crucial roles of miRNAs in lung cancer tumorigenesis and progression have been gradually recognized. MiR-1254, miR-574-5p and miR-21 are all significantly elevated in lung cancer (21). However, miR-146a and miR-1 expression were significantly decreased in patients with lung cancer (22,23). In addition, some miRNAs expression were closely associated with lymph node metastasis, such as miR-10b in lung cancer (24).
Thus far, studies on miR-1290 have been limited. Belian et al. (25) showed that miR-1290 participated in the process of gastric cancer cell resistance. miR-1290 was significantly up-regulated in clinical colon cancer tissue and it could impair cytokinesis and affect the reprogramming of colon cancer cells (26). However, miR-1290 expression levels in lung cancer are poorly characterized. In this study, the clinical significance of miR-1290 in NSCLC was firstly explored. We demonstrated that miR-1290 was significantly up-regulated in NSCLC tissues. In view of miR-1290 being encoded in the first intron of ALDH4A1 which is involved in metabolic signals (27), we detected the mRNA expression of ALDH4A1 in NSCLC tissues. However, there was no significant difference in ALDH4A1 mRNA expression between tumor tissues and NATs. Besides, there was also no significant correlation between ALDH4A1 mRNA expression and miR-1290.
Subsequently, we studied the association between miR-1290 expression levels and clinicopathological characteristics. Intriguingly, the up-regulation of miR-1290 was not only closely correlated with tumor TNM stage, but also correlated with lymph node metastasis. The results suggested that miR-1290 likely plays significant roles in NSCLC progression and metastasis. However, no significant association was examined between miR-1290 expression and tumor size. Thus, the statistical analysis could include a bias and the lymph node invasion could be the real correlated clinicopathological feature.
miRNAs are stable in blood and their expression signature are independent of age and sex (8,28). Pioneering studies on biomarkers have focused on circulating miRNAs (10,18,19). miRNA levels have been used as a novel non-invasive biomarker for the diagnosis and prognosis of various tumors. In this study, we found that serum miR-1290 expression was significantly higher in the NSCLC patients than in benign lung disease patients and healthy controls. Moreover, serum miR-1290 expression was closely correlated with lymph node metastasis and tumor TNM stage.
We also evaluated correlation between serum miR-1290 expression levels and prognosis of NSCLC. Kaplan-Meier survival curve analysis revealed that patients with high levels of serum miR-1290 in their pre-treatment serum had a significantly shortened overall survival. The median survival time of NSCLC patients with high-level expression of miR-1290 was only 12 months, whereas the median survival time of those with low levels of miR-1290 was 36 months. Univariate and multivariate Cox proportional hazards regression model analysis showed that high serum miR-1290 level was associated with an increased risk of death from NSCLC. Moreover, high expression of miR-1290 was an independent risk factor indicating poor prognosis for NSCLC patients. This was in agreement with studies reported by Li et al. (15) on pancreatic cancer.
At present, multiple miRNAs have been shown to promote or inhibit metastasis (29,30). Because metastasis is responsible for more than 90% of cancer-related deaths, it is important to define molecular mechanisms by which miRNAs regulate metastasis and define new therapeutic targets (31-33). Our present study showed that the expression of miR-1290 in NSCLC tissues and serum was closely correlated with lymph node metastasis. Additionally miR-1290 low expression levels were associated with better survival outcome, suggesting that its target could be a tumor suppressor gene. Bioinformatics analysis using microRNA.org (http://www.microrna.org/microrna/) and TargetScan (http://www.targetscan.org/) database revealed putative target genes of miR-1290. One of the most common predictive targets is lipoma preferred partner (LPP), of which a recent publication suggests a role in cell migration inhibition (34). Another interesting predictive target gene is suppressor of cytokine signaling 4 (SOCS4). SOCS4 is a known inhibitor of epidermal growth factor (EGF) receptor signaling, which is major determinant of epithelial cell proliferation, and due to its high oncogenic potential and incidence in cancer (35,36). SOCS4 could be a novel candidate for further exploration as a tumor-suppressor gene in gastric cancer (37). Therefore, further studies are indispensable to confirm whether LPP, SOCS4 or other related genes is possible target gene of miR-1290 in NSCLC.
In summary, high miR-1290 levels in NSCLC tissues and serum were demonstrated in our present study. High expression of miR-1290 closely correlated with lymph node metastasis, TNM stage, and poor prognosis. Furthermore, elevated serum miR-1290 was a meaningful independent prognostic marker for NSCLC. Further studies are needed to investigate the molecular mechanism of miR-1290 in NSCLC tumorigenesis and progression, and demonstrate that miR-1290 may be used as a potential therapeutic target for of NSCLC treatment.
Acknowledgements
We are grateful to the technical support from National Key Clinical Department of Laboratory Medicine of Jiangsu Province Hospital.
Funding: This work was supported by National Natural Science Foundation of China (No. 81371894) and Key Laboratory for Laboratory Medicine of Jiangsu Province of China (No. XK201114) and Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol 2011;6:49-69. [PubMed]
- Juergens R, Brahmer J. Targeting the epidermal growth factor receptor in non-small-cell lung cancer: who, which, when, and how? Curr Oncol Rep 2007;9:255-64. [PubMed]
- Waldman SA, Terzic A. Translating microRNA discovery into clinical biomarkers in cancer. JAMA 2007;297:1923-5. [PubMed]
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006;6):857-66.
- Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 2006;103:2257-61. [PubMed]
- Wulfken LM, Moritz R, Ohlmann C, et al. MicroRNAs in renal cell carcinoma: diagnostic implications of serum miR-1233 levels. PLoS One 2011;6:e25787. [PubMed]
- Heneghan HM, Miller N, Lowery AJ, et al. Circulating microRNAs as Novel Minimally Invasive Biomarkers for Breast Cancer. Ann Surg 2010;251:499-505. [PubMed]
- Mahn R, Heukamp LC, Rogenhofer S, et al. Circulating microRNAs (miRNA) in serum of patients with prostate cancer. Urology 2011;77:1265.e9-16.
- Huang Z, Huang D, Ni S, et al. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer 2010;127:118-26. [PubMed]
- Jakopovic M, Thomas A, Balasubramaniam S, et al. Targeting the epigenome in lung cancer: expanding approaches to epigenetic therapy. Front Oncol 2013;3:261. [PubMed]
- Cortinovis D, Monica V, Pietrantonio F, et al. MicroRNAs in non-small cell lung cancer: current status and future therapeutic promises. Curr Pharm Des 2014;20:3982-90. [PubMed]
- Morin RD, O'Connor MD, Griffith M, et al. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome Res 2008;18:610-21. [PubMed]
- Yelamanchili SV, Morsey B, Harrison EB, et al. The evolutionary young miR-1290 favors mitotic exit and differentiation of human neural progenitors through altering the cell cycle proteins. Cell Death Dis 2014;5:e982. [PubMed]
- Li A, Yu J, Kim H, et al. MicroRNA array analysis finds elevated serum miR-1290 accurately distinguishes patients with low-stage pancreatic cancer from healthy and disease controls. Clin Cancer Res 2013;19:3600-10. [PubMed]
- Lawrie CH, Gal S, Dunlop HM, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol 2008;141:672-5. [PubMed]
- Liu H, Du L, Wen Z, et al. Up-regulation of miR-182 expression in colorectal cancer tissues and its prognostic value. Int J Colorectal Dis 2013;28:697-703. [PubMed]
- Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008;10:1470-6. [PubMed]
- Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008;105:10513-8. [PubMed]
- Cho WC. OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer 2007;6:60. [PubMed]
- Foss KM, Sima C, Ugolini D, et al. miR-1254 and miR-574-5p Serum-Based microRNA Biomarkers for Early-Stage Non-small Cell Lung Cancer. J Thorac Oncol 2011;6:482-8. [PubMed]
- Jia Y, Zang A, Shang Y, et al. MicroRNA-146a rs2910164 polymorphism is associated with susceptibility to non-small cell lung cancer in the Chinese population. Med Oncol 2014;31:194. [PubMed]
- Nasser MW, Datta J, Nuovo G, et al. Down-regulation of micro-RNA-1 (miR-1) in lung cancer. Suppression of tumorigenic property of lung cancer cells and their sensitization to doxorubicin-induced apoptosis by miR-1. J Biol Chem 2008;283:33394-405. [PubMed]
- Roth C, Kasimir-Bauer S, Pantel K, et al. Screening for circulating nucleic acids and caspase activity in the peripheral blood as potential diagnostic tools in lung cancer. Mol Oncol 2011;5:281-91. [PubMed]
- Belian E, Kurucz R, Treue D, et al. Effect of YB-1 on the regulation of micro RNA expression in drug-sensitive and drug-resistant gastric carcinoma cells. Anticancer Res 2010;30:629-33. [PubMed]
- Wu J, Ji X, Zhu L, et al. Up-regulation of microRNA-1290 impairs cytokinesis and affects the reprogramming of colon cancer cells. Cancer Lett 2013;329:155-63. [PubMed]
- Pang S, Lynn DA, Lo JY, et al. SKN-1 and Nrf2 couples proline catabolism with lipid metabolism during nutrient deprivation. Nat Commun 2014;5:5048. [PubMed]
- Hunter MP, Ismail N, Zhang X, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One 2008;3:e3694. [PubMed]
- Taylor MA, Sossey-Alaoui K, Thompson CL, et al. TGF-beta upregulates miR-181a expression to promote breast cancer metastasis. J Clin Invest 2013;123:150-63. [PubMed]
- Tavazoie SF, Alarcon C, Oskarsson T, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 2008;451:147-52. [PubMed]
- Leidner RS, Ravi L, Leahy P, et al. The microRNAs, MiR-31 and MiR-375, as candidate markers in Barrett’s esophageal carcinogenesis. Genes Chromosomes Cancer 2012;51:473-9. [PubMed]
- Hötte GJ, Linam-Lennon N, Reynolds JV, et al. Radiation sensitivity of esophageal adenocarcinoma: the contribution of the RNA-binding protein RNPC1 and p21-mediated cell cycle arrest to radioresistance. Radiation Res 2012;177:272-9. [PubMed]
- Zhang T, Wang Q, Zhao D, et al. The oncogenetic role of microRNA-31 as a potential biomarker in oesophageal squamous cell carcinoma. Clin Sci (Lond) 2011;121:437-47. [PubMed]
- Kuriyama S, Yoshida M, Yano S, et al. LPP inhibits collective cell migration during lung cancer dissemination. Oncogene 2015. [Epub ahead of print]. [PubMed]
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 2006;7:505-16. [PubMed]
- Jones RB, Gordus A, Krall JA, et al. A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature 2006;439:168-74. [PubMed]
- Kobayashi D, Nomoto S, Kodera Y, et al. Suppressor of cytokine signaling 4 detected as a novel gastric cancer suppressor gene using double combination array analysis. World J Surg 2012;36:362-72. [PubMed]


