Expression and prognostic relevance of tumor carcinoembryonic antigen in stage IB non-small cell lung cancer
Abstract
Background: High serum carcinoembryonic antigen (CEA) levels have been reported to be associated with poor prognosis in non-small cell lung cancer (NSCLC), while the prognostic role of tumor CEA expression remains to be defined. The present study investigated the expression of tumor CEA in stage IB NSCLC, and correlated it with clinicopathological features and prognosis.
Patients and methods: Immunohistochemistry for tumor CEA was assessed in the specimens of 183 patients with stage IB NSCLC. Receiver-operating characteristic (ROC) curve analysis was used to determine the cut-off score for tumor positivity.
Results: High CEA expression was detected more frequently in adenocarcinomas (72.2%) and other NSCLCs (69.0%) than in squamous cell carcinomas (25.4%, P<0.001). Both univariate and multivariate analysis indicated that tumor CEA was an independent prognostic factor for overall and disease-free survival (P<0.05).
Conclusions: Elevated expression of tumor CEA may be an adverse prognostic indicator in stages IB NSCLC.
Key words: Non-small cell lung cancer; carcinoembryonic antigen; prognosis; receiver operating characteristic curve
Introduction
Lung cancer is the leading cause of cancer-related mortality around the world. Non-small cell lung cancer (NSCLC) represents more than 80% of all lung carcinomas (1). Although recent advances in technology have enabled earlier diagnoses, and advances in surgery, radiation therapy, imaging, and chemotherapy have produced improved responses rates, the clinical outcome of stage IB NSCLC is still unsatisfactory. The relevant 5-year survival rate remains no more than 70% despite surgery (2). In addition, routine use of adjuvant chemotherapy is now not justified for all patients with stage IB NSCLC, but a significant survival difference in favor of adjuvant chemotherapy may be present for the patients of subset who need to be defined (2). Thus, in order to further improve the survival rate of stage IB diseases, it is essential to explore and identify relevant biomarkers with adverse prognosis and modify the therapeutic strategy for these patients accordingly.
Carcinoembryonic antigen (CEA) is an oncofetal protein attached to epithelial cell apical membrane via its c-terminal glycosylphosphatidylinositol anchor, a member of the immunoglobulin superfamily of cell adhesion molecules (IgCAMs) (3). It is considered to involve in cell-cell recognition and modulate cellular processes (4). This biomarker has been extensively studied in a variety of neoplasms, such as colorectal (5,6), gastric (7,8), esophageal (9), pancreatic (10), and breast carcinoma (11,12), in regard to its potential role as a prognostic factor. For NSCLC in particular, serum CEA levels have been widely reported to be correlated with advanced disease (13,14), early relapse (15,16), pathological upstaging (17), poor therapeutic response (13,18) and survival (19,20). Nevertheless, to date, few data regarding CEA expression in lung cancer specimens are available, and the role of tumor CEA in NSCLC remains to be established. In the present study, therefore, we aimed to assess the expression of CEA in the primary lesions of stage IB NSCLC, and to elucidate its value in clinical prognosis.
Materials and methods
Patients
The study was approved by the Research Ethics Committee of the Cancer Center of Sun Yat-Sen University. From January 1992 to March 2004, we enrolled 183 consecutive patients with stage IB NSCLC who received surgical treatment with curative intent, and the resected specimens were assessed with immunohistochemistry (IHC) analysis. We verified and updated the survival data in the patient records through May 2009 using the database. Patients were selected based on the following eligibility criteria: (I) histopathologically proofed NSCLC; (II) disease stage was T2aN0M0 based on the seventh edition of the International Union Against Cancer (21). Staging system for Lung Cancer; (III) patients were at least 18 years of age, with no evidence of metastatic disease as determined by history, physical examination, and blood chemistry analysis or routine computed tomography; (IV) all patients received no adjuvant therapy. Patients were excluded based on the following criteria: history of previously treated cancer other than basal or squamous cell carcinoma of the skin or with preoperative chemotherapy and/or radiotherapy. The demographic and clinicopathological parameters of the 183 patients are listed in Table 1.
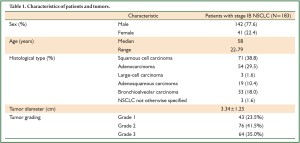
Full Table
Immunohistochemistry
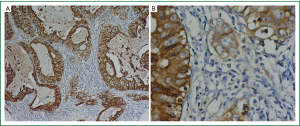
Immunoperoxidase stain for CEA (ZCEA1; 1:100 dilution; Fuzhou Maxim Inc., Fuzhou, Fujian, China) was done on 4 μm-thick paraffin sections. The slides were deparaffinized in xylene then hydrated prior to antigen retrieval by microwaving in sodium citrate buffer (pH 6.0). The slides were then incubated with a peroxidase block, followed by the primary antibody. After a PBS wash, the slides were incubated with the secondary antibody and 3,3'-diaminobenzidine. The peroxidase block, secondary antibody and 3,3'-diaminobenzidine were from the DakoCytomation EnVision System (Glostrup, Denmark). After a hematoxylin counterstain (Hematoxylin 7211; Richard-Allen Scientific, Kalamazoo, Michigan, United States of America), the slides were coverslipped (Figure 1).
IHC scoring
A positive control sample was evaluated with each batch of slides. Each slide was assigned a score: the score of tumor cells staining multiplied by the score of staining intensity. Tumor cell staining was assigned a score using a semiquantitative six-category grading system: 0, none of tumor cells staining; 1, 1% to 10% of tumor cells staining; 2, 11% to 25% of tumor cells staining; 3, 26% to 50% of tumor cells staining; 4, 51% to 75% of tumor cells staining; 5, more than 75% of tumor cells staining. Stain intensity was assigned a score using a semiquantitative four-category grading system: 0, non-staining; 1, weak staining; 2, moderate staining; 3, strong staining. Two experienced pathologists independently scored 400 NSCLC samples including the cases used in this study blinded to clinical follow-up data. The complete score agreement between these two pathologists is 89% of the cases, indicating that the scoring method was highly reproducible. The third pathologist intervened and evaluated the cases with different IHC scores. The score was selected when the third pathologist agreed with one of previous pathologists. For the cases with three different score, the three pathologists would review these cases together to reach an agreement.
Selection of cut-off score
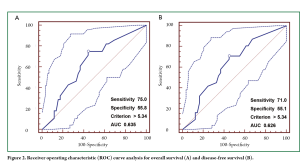
The cut-off scores for tumor CEA expression were selected based on receiver operating characteristic (ROC) curve analysis (22-24). The sensitivity and specificity for the outcome under study were plotted, thus generating an ROC curve. The score closest to the point with both maximum sensitivity and specificity [i.e. the point (0.0, 1.0) on the curve] was selected as the cut-off score leading to the greatest number of tumors correctly classified as having or not having the clinical outcome. The area under the ROC curve (AUC) was calculated to estimate the discriminatory power of tumor CEA over the entire range of scores for overall and disease-free survival. Both generation and analysis of the ROC curve were performed by MedCalc statistical software package 11.0.1 (MedCalc Software bvba, Belgium).
Statistical analysis
Associations between categorical variables were analyzed using Chi-square test. Survival curves were calculated by the Kaplan-Meier method and were compared by the log-rank test. Time to event (death or relapse) was calculated from date of surgery to date of event. In event-free subjects, the time variable was censored at date of last follow-up. Multivariate analysis of prognostic factors was performed using Cox’s regression model. A significant difference was declared if the p value from a two-tailed test was less than 0.05. All of the statistical analysis was performed using the SPSS 13.0 for Windows software system (SPSS Inc, Chicago, IL).
Results
Expression of tumor CEA
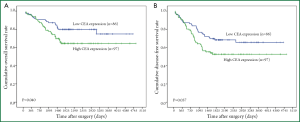
According to the ROC curves for overall survival (OS) and disease-free survival (DFS) analyzed in our study (Figure 2), threshold value of 5.34 was the closest to the point with both maximum sensitivity and specificity, and thereby was selected as the cut-off score. Therefore, the expression of tumor CEA assessed in the 183 stage IB NSCLCs could be categorized into two groups, namely, high expression and low expression groups. The enhanced expression of CEA was found in 97 cases (53%), whereas decreased CEA expression was detected in 86 cases (47%).
Correlation between tumor CEA and clinicopathological features
The association between tumor CEA expression and clinicopathological variables of stage IB NSCLC is shown in Table 2. High CEA expression was found in 25.4% of squamous cell carcinomas (SCCs), significantly lower than that of adenocarcinomas (ADKs) and other NSCLCs (72.2% and 69.0%, respectively). There were no significant correlations between tumor CEA and other clinicopathological parameters including gender, age, tumor diameter and grading.
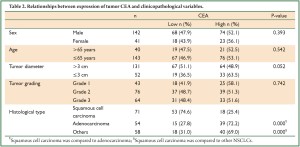
Full Table
The impact of tumor CEA expression on overall and disease-free survival in NSCLC
At the time of this analysis (May 2009), with a median follow-up of 1972 days (range 91-4,842 days), 133 patients (72.7%) were alive and 111 patients (60.7%) remained free of disease. The 1-year and 5-year overall survival probabilities for the entire group were 89% and 73% respectively. And 1-year and 5-year disease-free survival rates were 82% and 62% respectively. The Kaplan-Meier survival curves (Figure 3) showed that patients with low CEA expression experienced significantly better 5-year OS and DFS than those with high CEA expression (80% vs. 64%, P=0.040; 68% vs. 52%, P=0.037, respectively). Nevertheless, in further stratified analysis split by histological subtypes, the expression of tumor CEA had no significant impact on OS and DFS of patients with SCCs (P=0.146 and P=0.289), ADKs (P=0.402 and P=0.126) or other NSCLCs (P=0.495 and P=0.518).
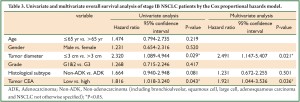
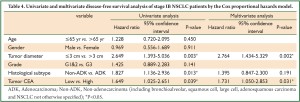
We performed analysis using the Cox proportional hazards model to identify factors involved in OS (Table 3) and DFS (Table 4) of stage IB NSCLC patients. The univariate analysis revealed that tumor diameter and tumor CEA expression were significant prognostic indicators for OS, while tumor diameter, histological subtype and tumor CEA expression were of prognostic significance for DFS. In further multivariate analysis where tumor diameter, histological subtype and tumor CEA expression were taken into account, tumor CEA level was confirmed to be an independent prognostic factor for both OS and DFS. Another parameter related to shortened OS and DFS was tumor diameter.
Full Table
Full Table
Discussion
To date, numerous studies have been performed to elucidate the prognostic role of serum CEA in NSCLC; however, the expression pattern of tumor CEA and its potential involvement in the formation and progression of NSCLC still await clear-cut demonstration. One problem faced by researchers is the determination of the extent of tumor immunohistochemical positivity for CEA which is clinically and biologically relevant. Several existing studies had applied different scoring systems and predetermined cut-off scores which might be set arbitrarily (25-28). This may primarily be responsible for the contradictory results of these studies evaluating tumor CEA and the difficulty in ascertaining its prognostic value in NSCLC. Therefore, our study utilized a reproducible scoring method which took both staining percentage and intensity into account, and selected the cut-off score based on ROC curve analysis so that the trade-off between sensitivity and specificity was the smallest, leading to the greatest overall number of correctly classified tumors with and without the clinical outcome (22-24).
The main finding of the present study is that in radically resected stage IB NSCLCs, low expression of tumor CEA was significantly correlated with better OS and DFS, both by univariate and multivariate analysis. This result was inconsistent with previous reports (25,28). Apart from different immunohistochemical scoring systems used and threshold values selected, it should be noted that lung carcinomas of all stages and stage I-II were involved in the studies of Linnoila et al. (25) and Veronesi et al. (28) respectively, while our study focused on a more homogeneous population (all patients with pathological stage IB disease). Different therapeutic strategies, like adjuvant chemotherapy and radiotherapy taken in advanced diseases, could make the comparison between their findings and ours impractical. Furthermore, CEA is a member of the IgCAM superfamily and can mediate homotypic binding to itself or heterotypic binding to one of several other CEACAM family members and thereby mediate cell-cell adhesion (4). Thus, it is reasonable to predict that tumors with high CEA expression, on the one hand, could possess an increased capacity to metastasis due to vascular-tumoral cell-cell adhesion processes; on the other hand, its over-expression in the cancer tissues might also promote the cohesion of tumor cells and prevent them from migration and metastasis. Obviously, the critical issue lies in which physiopathological process takes the advantage under different circumstances, such as different tumor types in different stages. Since supportive evidence is lacking, more relevant studies are needed to confirm this hypothesis. Interestingly, Veronesi et al. also found that the prognostic significance of tumor CEA was solely present in SCC in stratified analysis by histology (28), which was not in line with our finding. In fact, we observed an opposite tendency, although it did not reach statistical significance, that declined tumor CEA expression was associated with better OS in SCC (P=0.146) but with better DFS in ADK (P=0.126). We realized that inadequate number of patients for analysis after stratification might be the reason of the inability to verify the trend. Therefore, it is worth enhancing statistical power to obtain a clearer picture in the future.
Carcinoembryonic antigen, a glycoprotein expressed during early fetal life, is the product of the CEACAM5-gen. Its expression is restricted to epithelial cells, and it is found more abundantly on apical surface of gastrointestinal epithelium, but can also be found in other mucosal epithelia, such as lung (3,4). Our study also found that high tumor CEA levels were much more frequent in ADK (72.2%) than in SCC (25.4%), which was in agreement with previous studies (27,28). Similarly, other series had indicated that elevated serum CEA was more prevalent in ADK than in SCC (19,28,29). In the researches of Okamura et al. (29) and Veronesi et al. (28), a significant correlation between serum and tumor CEA had also been identified, and this relation persisted only in ADK after stratification by histology. All these findings raise the possibility that heterogeneous expression of tumor CEA might occur at a relatively early stage, and provide a tumorigenic contribution to lung adenocarcinoma. Therefore, further analysis of CEA gene and gene product at different stages in the progression of lung adenocarcinoma might provide more clues in this regard. In summary, the present study suggests that high tumor CEA expression might be an adverse prognostic factor for the patients with stage IB NSCLC. However, further studies with larger cohorts of patients are required to verify this, especially in view of the contradictory results of few other published studies in this area. Patients with elevated tumor CEA expression may benefit from adjuvant therapy; but further series with randomization and longer follow-up are needed for the establishment of a safe and effective management plan.
Acknowledgements
This study was supported by the Fundamental Research Funds for the Central Universities and the grant from Youth Training Plan of Sun Yat-Sen University (No. 10ykpy38), the Research Award Fund for Outstanding Young Researchers in Sun Yat-Sen Can-cer Center (Nos. 3030451720 06 and 3030 45172005), the National Natural Science Foundation of China (No. 30901728) and the Science & Technology Pillar Program of Guangdong Province (No. 2011B031800220). The authors declare their independence of sponsors, and the content of this article has not been influenced by the sponsors.
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96.
- Hennon MW, Demmy TL. Video-assisted thoracoscopic surgery (VATS) for locally advanced lung cancer. Ann Cardiothorac Surg 2012;1:37-42.
- Kokkonen N, Ulibarri IF, Kauppila A, et al. Hypoxia upregulates carcinoembryonic antigen expression in cancer cells. Int J Cancer 2007;121:2443-50.
- Kuespert K, Pils S, Hauck CR. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol 2006;18:565-71.
- Li M, Li JY, Zhao AL, et al. Comparison of carcinoembryonic antigen prognostic value in serum and tumour tissue of patients with colorectal cancer. Colorectal Dis 2009;11:276-81.
- Hatate K, Yamashita K, Hirai K, et al. Liver metastasis of colorectal cancer by protein-tyrosine phosphatase type 4A, 3 (PRL-3) is mediated through lymph node metastasis and elevated serum tumor markers such as CEA and CA19-9. Oncol Rep 2008;20:737-43.
- Tamura N, Iinuma H, Takada T. Prospective study of the quantitative carcinoembryonic antigen and cytokeratin 20 mRNA detection in peritoneal washes to predict peritoneal recurrence in gastric carcinoma patients. Oncol Rep 2007;17:667-72.
- Han SU, Kwak TH, Her KH, et al. CEACAM5 and CEACAM6 are major target genes for Smad3-mediated TGF-beta signaling. Oncogene 2008;27:675-83.
- Mroczko B, Kozlowski M, Groblewska M, et al. The diagnostic value of the measurement of matrix metalloproteinase 9 (MMP-9), squamous cell cancer antigen (SCC) and carcinoembryonic antigen (CEA) in the sera of esophageal cancer patients. Clin Chim Acta 2008;389:61-6.
- Kelly KJ, Wong J, Gladdy R, et al. Prognostic Impact of RT-PCR-Based Detection of Peritoneal Micrometastases in Patients with Pancreatic Cancer Undergoing Curative Resection. Ann Surg Oncol 2009;16:3333-9.
- Nicolini A, Tartarelli G, Carpi A, et al. Intensive post-operative follow-up of breast cancer patients with tumour markers: CEA, TPA or CA15.3 vs. MCA and MCA-CA15.3 vs. CEA-TPA-CA15.3 panel in the early detection of distant metastases. BMC Cancer 2006;6:269.
- Charalabopoulos K, Kotsalos A, Batistatou A, et al. Selenium in serum and neoplastic tissue in breast cancer: correlation with CEA. Br J Cancer 2006;95:674-6.
- Chiu CH, Shih YN, Tsai CM, et al. Serum tumor markers as predictors for survival in advanced non-small cell lung cancer patients treated with gefitinib. Lung Cancer 2007;57:213-21.
- Shi HZ, Liang QL, Jiang J, et al. Diagnostic value of carcinoembryonic antigen in malignant pleural effusion: a meta-analysis. Respirology 2008;13:518-27.
- Sakao Y, Tomimitsu S, Takeda Y, et al. Carcinoembryonic antigen as a predictive factor for postoperative tumor relapse in early-stage lung adenocarcinoma. Eur J Cardiothorac Surg 2004;25:520-2.
- Kashiwabara K, Saeki S, Sasaki J, et al. Combined evaluation of postoperative serum levels of carcinoembryonic antigen less than or equal to 2.5 ng/mL and absence of vascular invasion may predict no recurrence of stage I adenocarcinoma lung cancer. J Thorac Oncol 2008;3:1416-20.
- Yamazaki K, Yoshino I, Yohena T, et al. Clinically predictive factors of pathologic upstaging in patients with peripherally located clinical stage IA non-small cell lung cancer. Lung Cancer 2007;55:365-9.
- Ardizzoni A, Cafferata MA, Tiseo M, et al. Decline in serum carcinoembryonic antigen and cytokeratin 19 fragment during chemotherapy predicts objective response and survival in patients with advanced nonsmall cell lung cancer. Cancer 2006;107:2842-9.
- Sawabata N, Maeda H, Yokota S, et al. Postoperative serum carcinoembryonic antigen levels in patients with pathologic stage IA nonsmall cell lung carcinoma: subnormal levels as an indicator of favorable prognosis. Cancer 2004;101:803-9.
- Okada M, Nishio W, Sakamoto T, et al. Prognostic significance of perioperative serum carcinoembryonic antigen in non-small cell lung cancer: analysis of 1,000 consecutive resections for clinical stage I disease. Ann Thorac Surg 2004;78:216-21.
- Lombardi R, Cuicchi D, Pinto C, et al. Clinically-staged T3N0 rectal cancer: is preoperative chemoradiotherapy the optimal treatment? Ann Surg Oncol 2010;17:838-45.
- Hanley JA. Receiver operating characteristic (ROC) methodology: the state of the art. Crit Rev Diagn Imaging 1989;29:307-35.
- Zlobec I, Steele R, Terracciano L, et al. Selecting immunohistochemical cut-off scores for novel biomarkers of progression and survival in colorectal cancer. J Clin Pathol 2007;60:1112-6.
- Zhu ZH, Sun BY, Ma Y, et al. Three immunomarker support vector machines-based prognostic classifiers for stage IB non-small-cell lung cancer. J Clin Oncol 2009;27:1091-9.
- Linnoila RI, Piantadosi S, Ruckdeschel JC. Impact of neuroendocrine differentiation in non-small cell lung cancer. The LCSG experience. Chest 1994;106:367S-371S.
- Ford CH, Stokes HJ, Newman CE. Carcinoembryonic antigen and prognosis after radical surgery for lung cancer: immunocytochemical localization and serum levels. Br J Cancer 1981;44:145-53.
- Graziano SL, Tatum AH, Newman NB, et al. The prognostic significance of neuroendocrine markers and carcinoembryonic antigen in patients with resected stage I and II non-small cell lung cancer. Cancer Res 1994;54:2908-13.
- Veronesi G, Pelosi G, Sonzogni A, et al. Tumour CEA as predictor of better outcome in squamous cell carcinoma of the lung. Lung Cancer 2005;48:233-40.
- Okamura A, Ohkawa J, Fujisawa H, et al. Clinicopathological study on the relationship between serum-CEA and tissue-CEA of resected lung cancer cases. Acta Pathol Jpn 1984;34:1209-19.


