Unusual causes of pneumothorax
Introduction
Spontaneous pneumothorax may occur in association with various diseases of the lung. Several uncommon pulmonary conditions are frequently associated with pneumothorax, such as lymphangioleiomyomatosis (LAM) and pulmonary Langerhans’ cell histiocytosis (PLCH). On the other hand, there are common systemic diseases that are infrequently or rarely associated with pneumothorax. Examples of the latter group include: anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis, systemic lupus erythematosis (SLE), rheumatoid arthritis and related conditions, scleroderma, dermatomyositis, and inflammatory bowel disease. Finally, extremely rare conditions such as Birt-Hogg-Dube syndrome have an association with pneumothorax. This chapter will provide a review of the evaluation and management of patients with these unusual causes of pneumothorax.
Lymphangioleiomyomatosis (LAM)
LAM is a rare disease that is associated with cystic lung disease, angiomyolipomas, and obstruction of the lymphatic system (1-3). Currently thought to be a neoplastic disorder (4), the condition has a marked female predominance. LAM is related in pathophysiology and clinical presentation to the Tuberous Sclerosis Complex (TSC) syndrome. TSC differs from LAM in that TSC is an autosomal dominant genetic disorder associated with neoplastic lesions in multiple organs including angiomyolipomas and angiofibromas (5). The first reported case of LAM was a patient with bilateral spontaneous (6). Pneumothorax has been reported to precede the diagnosis of LAM in 82% of patients who have had at least one pneumothorax (7). Pneumothorax is the second most common clinical feature of LAM, after dyspnea (8). On average, patients with LAM have a lifetime incidence of 3.5 episodes of pneumothorax (7).
In patients with LAM, foci of smooth muscle cells, known as LAM cells, infiltrate the lung parenchyma as well as associated lymphatics and blood vessels, and are associated with the macroscopic cystic lesions (Figure 1). All LAM cells stain positively for smooth muscle actin, vimentin, and desmin (1). LAM cells frequently stain positively with the monoclonal antibody HMB-45, which targets premelanosomal protein gp-100 (9). The HMB-45 stain is useful diagnostically in patients being evaluated for LAM, because other smooth muscle cells within the lung do not stain with this agent. LAM cells also may have estrogen and progesterone receptors present, which is a finding not seen in normal lung tissue (10).
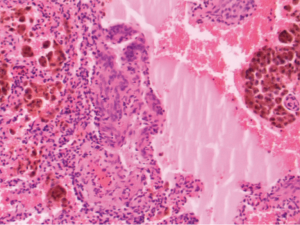
The pathologic changes that are present in the lungs of patients with LAM provide insight concerning the mechanism by which pneumothorax occurs. Large, cystic airspaces are demonstrated within the lung parenchyma which would lead to pneumothorax upon rupture. Theories for the development of the cystic air spaces have included obstruction of conducting bronchi by LAM cells, or destruction of the supporting interstitial matrix by enzymes elaborated by LAM cells (11). Cysts involving the pleural surface are common, and their rupture into the pleural space would lead to pneumothorax (11).
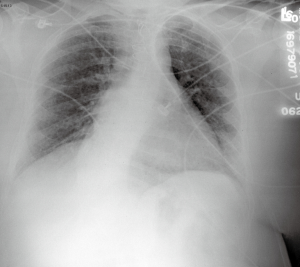
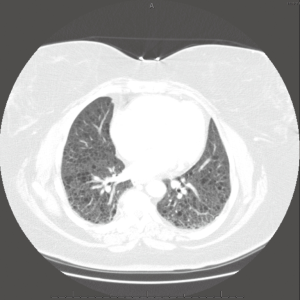
The macroscopic cystic lesions which are seen in pathological sections of the lung in patients with LAM are associated with abnormalities seen in such patients on chest radiography and in computed tomography (CT) scans of the chest. Patients with LAM tend to have increased lung volumes compared to normal on routine chest radiography, a finding which distinguishes LAM from other interstitial pneumonitides. Patients often have reticulonodular interstitial infiltrates that are symmetric and diffuse. Cystic changes are present in patients with advanced disease (Figure 2). Pneumothorax may be visible in the appropriate patient context. CT scan of the chest demonstrates cysts of the lung which are round, thin-walled, and bilateral (Figure 3). Other CT findings include ground glass opacities, mediastinal adenopathy, and pleural effusions (12).
Patients with TSC have inactivating mutations in either of two genetic loci, TSC1 on chromosome 9q34 (13) or TSC2 on chromosome 16p13 (14). These loci code for the cell growth regulating proteins hamartin and tuberin, respectively. The loss or inactivation of these two proteins results in inappropriate cell growth with dysregulated protein synthesis and cellular motility (15). Research has suggested that patients with LAM may have an acquired disorder which may affect the synthesis, structure, or function of these proteins. Preliminary work by Carsillo and colleagues suggests that at least some patients with LAM have TSC2 mutations in lung and kidney tissue affected by the disease process in LAM (16). This same work demonstrated that the specific mutations identified were conserved within individuals, suggesting that the LAM cells represent a clonal, neoplastic cell cluster. The concept that LAM is a neoplastic disorder is further supported by the fact that recurrence of LAM in lung transplant recipients has been demonstrated to be due to a recipient clone of LAM cells in two cases (17,18).
Much of the data concerning the epidemiology of LAM and associated pneumothorax is derived from case series. The incidence of pneumothorax in LAM is very high, with reported rates between 39-76%. Early work by Corrin and colleagues reviewed a small group of 28 patients (including 23 previously unreported cases) and demonstrated an incidence of pneumothorax of 43% (19). Taylor and associates conducted a retrospective review of the clinical course of 32 patients with LAM followed for an average of 8.5 years at two centers, and found that the pneumothorax incidence in their series was 53% at the time of onset of disease, and that 81% of the patients suffered pneumothorax during the follow-up period (20). Kitaichi and coworkers studied 46 patients from Japan, Korea, and Taiwan, finding an incidence of pneumothorax of 39% (12). A group at the National Institutes of Health prospectively evaluated 35 women with LAM, finding a pneumothorax incidence of 69% (21). The highest incidence in any case series was reported by Oh and coworkers in Korea who studied 21 patients finding a pneumothorax incidence of 76% (22). Pneumothorax rates from case series in France (46%) and the United Kingdom (60%) have also been high (8,23). The largest series is that derived from the National Heart, Lung, and Blood Institute’s LAM registry demonstrating an incidence of 56% in 230 patients (24).
A striking clinical feature of LAM is that patients have a very high recurrence rate of pneumothorax. In a study of 193 patients who enrolled in the LAM Foundation data base, who had a history of pneumothorax, and who provided complete information, 140 patients (73%) had recurrent pneumothorax (7). The recurrence was ipsilateral in 71% and contralateral in74% of patients. Among patients having recurrent pneumothorax, the average number of pneumothoraces was 4.5.
There have been case reports of the occurrence of bilateral pneumothorax in patients with LAM. The first case reported was a patient who presented with bilateral pneumothorax (6). Numerous other cases have been reported since that time (25-29). In their review of the LAM Foundation registry, Almoosa and colleagues found that 8 of 193 patients (4%) had suffered bilateral pneumothoraces (7). This complication may have life-threatening implications, as tension pneumothorax has been described (25).
The best data concerning treatment of pneumothorax in LAM is presented in the retrospective review of the LAM Foundation registry by Almoosa and colleagues (7). Of 676 reported instances of pneumothorax, 117 were observed, simple aspiration was performed in 39, small-bore chest tubes placed in 42, tube thoracostomy was performed in 481, chemical pleurodesis was performed in 209, and surgery in 191. The most common pleurodesis agent used was talc. Recurrence occurred frequently in conservatively managed patients (66%), but less commonly in those patients treated with chemical pleurodesis (27%) or surgical pleurodesis (32%). Patients who had been treated with pleurodesis and subsequently received lung transplantation were more likely (29% vs. 3%) to have bleeding complications during surgery. In a smaller group of patients, Johnson and Tattersfield examined pneumothorax treatment outcomes (23). Thirty patients had 83 pneumothoraces. Fifty-two pneumothorax episodes were treated “non-surgically” (observation, aspiration, tube thoracostomy) with a recurrence rate of 62%. Thirty-one pneumothorax episodes were treated “surgically” (pleural abrasion, pleurodesis, pleurectomy, thoroscopic pleurodesis, bullectomy plus pleurodesis) with a recurrence rate of 12.9%. Although expert consensus is that patients with an initial secondary spontaneous pneumothorax should be aggressively managed (30), surveys of patients with LAM suggest that such patients prefer conservative management in lieu of pleurodesis for an initial pneumothorax (31). Only 25% of LAM patients responding to a survey thought that pleurodesis for an initial pneumothorax was appropriate, whereas 60% would accept pleurodesis for recurrent episodes (31).
Because of the frequent occurrence of pneumothorax in LAM, concerns have been raised about the advisability of air travel for these patients. This concern stems from knowledge of Boyle’s Law, which predicts the increase in volume of a fixed quantity of gas under the circumstances of decreasing pressure. Should the volume of a cyst in the lung expand under the conditions of decreased ambient pressure in an aircraft cabin, then rupture of the cyst and pneumothorax may be likely to occur. Survey data from 327 patients with LAM has indicated that 35% of patients report being advised by their physicians not to travel by air (32). The same survey reports eight confirmed episodes of pneumothorax among 454 flights taken by 276 women (32). From a population of 281 patients with LAM, Taveira-DaSilva and co-workers found that LAM patients have a risk of pneumothorax of 1.1 for every 100 flights (33). Given the potential catastrophic consequences of pneumothorax and the limited ability to diagnose and treat this problem during a commercial aircraft flight, this author would caution most LAM patients to avoid air travel.
Concerns exist that LAM patients may have poor pregnancy outcomes and that their condition may be worsened by pregnancy (1,11). Case series describe the occurrence of pneumothorax during pregnancy raising suspicion that pregnancy may predispose to this problem (22,23). In each series, three patients had pneumothoraces while pregnant. Cohen and co-workers obtained survey data from 328 patients with LAM (34). Of the 328 women, 123 (37.3%) had never been pregnant, with over half avoiding pregnancy at least in part because of medical concerns. Most of the remaining patients (178 out of 205) had pregnancies before the diagnosis of LAM and had generally good pregnancy outcomes. However, five percent of this group had suffered pneumothorax during their pregnancy possibly suggesting that LAM was already present. Pneumothorax rates were high in patients who were diagnosed with LAM prior to pregnancy (four pneumothoraces among 15 pregnancies) or who had LAM diagnosed concurrently with pregnancy (10 pneumothoraces among 15 pregnancies). Although the relationship between LAM and pneumothorax during pregnancy has not been systematically studied, it seems prudent to counsel patients with LAM that they may be at increased risk of pneumothorax during pregnancy.
Treatment of patients with LAM who present with a pneumothorax should include either tube thoracostomy with pleurodesis or a surgical approach with mechanical or chemical pleurodesis to prevent recurrence. Observation alone, or tube thoracostomy without chemical pleurodesis, are associated with higher pneumothorax recurrence rates than more aggressive approaches. Patients should be advised of the risks associated with air travel and pregnancy. A discussion of systemic treatment for LAM is beyond the scope of this chapter. Progesterone has been shown to be ineffective in treating LAM (35). Lung transplantation has been performed in patients with LAM with survival rates equal to, or better than, other disease states (1). Clinical trials are underway with novel agents targeting the pathophysiologic basis for the disease (1,3).
PLCH
PLCH is a rare disease that is also referred to as Histiocytosis X and Eosinophilic Granuloma. This condition represents one of a group of disorders generally referred to as Langerhans cell histiocytosis which range in severity from isolated lesions of the bone (unifocal Langerhans cell histiocytosis) to disseminated multi-system disease (36). Generally considered to be a disease of smokers, there have been occasional reports of patients developing the condition who do not smoke cigarettes (37). PLCH occurs most frequently in persons who are white and between the third and fifth decades of life (37,38). Approximately 5% of patients who have interstitial lung disease and who have a diagnosis made by biopsy have PLCH, according to one large series (39). However, in many patients the diagnosis is made clinically without a biopsy being obtained (40,41). Recently this condition has recently been extensively reviewed (37,40,41).
The link between smoking and PLCH is particularly strong (37,40,41). This author and others have often noted that patients with PLCH are especially heavy smokers who usually have trouble quitting (40). Zeid and Muller demonstrated in an experimental model that mice with passive exposure to cigarette smoke had a 20-fold increase in Langerhans dendritic cells (42). The mice also developed interstitial peribronchial granuloma. Cessation of the exposure led to a decrement in the number of Langerhans cells and granuloma, although some inflammatory changes persisted. Because systemic multi-organ Langerhans’ cell histiocytosis appears to be a clonal and potentially neoplastic process, Yousem and co-workers investigated the clonality of lesions associated with PLCH (43). These investigators report that the lesions of PLCH represent a non-clonal proliferation of Langerhans’ cells and therefore may represent a reactive process to cigarette smoking. Bernstrand and associates described two individuals who were diagnosed with Langerhans’ cell histiocytosis as children (44). In each case, the pulmonary manifestations did not occur until adulthood after the individuals had been smoking for 3 years. Investigations conducted by a group at the National Institutes of Health using bronchoalveolar lavage in small groups of smoking and non-smoking subjects revealed that smokers had an increase in the population of airways Langerhans’ cells compared to non-smokers (45). Pathologic lesions other than PLCH thought to be associated with tobacco use and smoking, to include respiratory bronchiolitis and desquamative interstitial pneumonia, have been found to be present concurrently in patients with PLCH (46). Two patients with PLCH who received lung transplants had their disease recur upon resumption of smoking (47). Finally, radiographic and clinical improvement has been demonstrated after smoking cessation (48). Although most patients with PLCH have a history of smoking, there have been occasional exceptions (37). Familial associations have been reported (49).
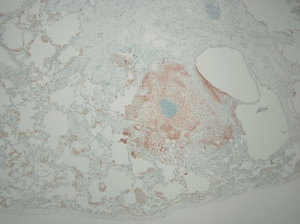
The lungs of patients with less advanced manifestations of PLCH demonstrate surface cystic structures and nodules upon gross examination, while more advanced cases are characterized by hyperinflation and prominent cystic changes (50,51). There is a tendency for greater involvement of the upper lobes. Histologic sections demonstrate loosely formed granulomatous nodules associated with airways (Figure 4). The nodules are formed by a variety of inflammatory cells to include Langerhans’ cells. In more advanced disease, cystic lesions and fibrotic scars are seen (50-52). Immunohistochemical staining demonstrates the cell surface antigens S-100 and CD1a on the Langerhans’ cells (52). The mere presence of Langerhans’ cells on histopathologic section is not alone sufficient for the diagnosis as they may be present in other disease states (53,54). The appropriate pattern of pathologic change must be observed in addition to the presence of Langerhans’ cells for the diagnosis of PLCH to be confirmed.
Chest radiographs in patients with PLCH reveal diffuse reticulonodular infiltrates that are symmetric (Figure 5). The lung bases may be spared, and cystic changes may be present (55). Unlike other interstitial pneumonitides, the lung volumes may be normal or increased (55,56). High resolution CT scan (HRCT) is very useful in the evaluation of PLCH. HRCT commonly demonstrates nodules and cysts that involve the upper lobes of the lung (Figure 6) (57-59). Sparing of the lung bases is also a frequent finding (Figure 7). As the disease advances, the nodules and cysts tend to coalesce (60). Patients with advanced disease may have highly distorted architecture with cystic changes. As these patients are usually smokers, features of other smoking-related lung diseases such as emphysema, diffuse interstitial pneumonitis, and respiratory bronchiolitis may also be seen. It may be possible to make a presumptive diagnosis of PLCH in the appropriate clinical setting with characteristic chest radiographs (40).
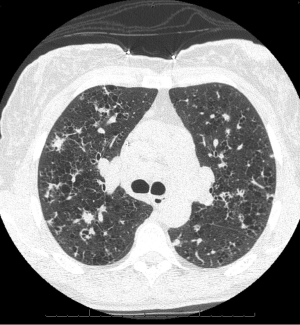
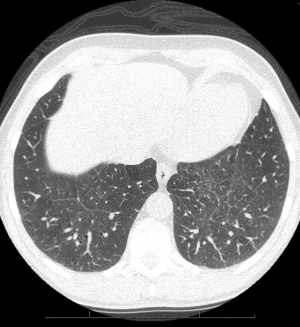
Spontaneous pneumothorax has long been known to be a complication of PLCH (61). Reports of the frequency of spontaneous pneumothorax in PLCH have noted an incidence rate between 10-20%, depending on the series, with the most common symptoms being dyspnea and chest pain (62). Pneumothorax is attributed to the rupture of subpleural cysts (37,62,63). Pneumothorax may recur in patients with PLCH, a circumstance that may require surgical treatment (62,64). Early case series of patients with PLCH have noted that pneumothorax is a frequent occurrence and have studied the histopathology, radiographic findings, and pulmonary physiology of this disease (50,56,65,66). Delobbe and co-workers studied 45 patients with PLCH (67). They found that 16% of their patients had pneumothorax. Although they studied risk factors for poor outcome, pneumothorax was not a specific risk factor studied. Vassallo and colleagues reviewed the Mayo Clinic experience with PLCH (37). In their large series of 102 patients, 12 had pneumothoraces. Pneumothorax was not identified as a mortality risk factor among the variables that were studied. Two years later, the Mayo Clinic group reported extended results specifically concerning pneumothorax in the same group of 102 patients (63). Four additional patients were reported to have suffered pneumothorax, for a total of 16 patients. Among these 16 patients, there had been 37 pneumothoraces, confirming that some patients have recurrent episodes of this problem. Pulmonary function tests and survival were not different between those patients suffering pneumothorax and other patients with PLCH. Patients with pneumothorax had a 58% rate of ipsilateral recurrence if the pneumothorax was managed by observation or with a chest tube without pleurodesis. Following surgical management of pneumothorax, the group did not report any recurrences.
In summary, pneumothorax is common in patients with PLCH. The occurrence of pneumothorax in patients with PLCH does not appear to effect pulmonary physiology or overall outcomes. An episode of pneumothorax in a patient with PLCH carries a high risk of recurrence even if treated with a chest tube if sclerosing agents are not used. In concert with previous consensus statements (68), this author recommends surgical treatment of patients with PLCH following the first occurrence of pneumothorax. Surgical interventions such as mechanical pleurodesis or talc insufflation via thoracoscopy seem particularly amenable for these patients (63,68). Partial pleurectomy has also been utilized. As is the case for all PLCH patients, smoking cessation should be encouraged.
Systemic diseases uncommonly associated with pneumothorax
A number of systemic inflammatory diseases have been rarely associated with pneumothorax. Most of the data concerning these conditions is described in small case series and case reports.
ANCA-associated vasculitis
ANCA-associated vasculitis is associated with a variety of pulmonary manifestations, among which is the occurrence of spontaneous pneumothorax. Belhassen-Garcia and colleagues reported a case of pneumothorax associated with Wegener Granulomatosis and reviewed the literature available for this problem (69). They determined that there have only been 21 cases of spontaneous pneumothorax reported with this condition, and estimated that the incidence was less than 5%. Sezer and co-workers reported a case of pneumothorax that resulted from rupture of a sub-pleural cavitated nodule (70). Storelli and others reported an unusual case of pneumothorax associated with Wegener’s Granulomatosis that they attributed to pleural scarring resulting from immunosuppressive therapy (71). Other reported cases have also been associated with rupture of cavitary lesions and immunosuppressive therapy (72-78).
SLE
SLE has been associated with pneumothorax, including bilateral and recurrent pneumothoraces. The entity is rare, with fewer than a dozen cases having been reported. Noted associations have included cystic lung disease, pleural disease, and corticosteroid therapy. Sawkar and Easom reported a case of a 27-year-old woman with SLE who had cystic lung disease and recurrent spontaneous pneumothorax (79). Richards and co-workers described a case of a 34-year-old woman with a diagnosis of SLE who presented with diffuse pulmonary parenchymal infiltrates as well as cough and shortness of breath (80). She was treated with prednisone and antibiotics. Four weeks later, the patient developed bilateral pneumothoraces. She was treated with bilateral chest tubes. Two weeks after this treatment, the patient had a cardiac arrest and expired. Autopsy did not reveal any specific findings predisposing this patient to pneumothorax other than pulmonary fibrosis. Masuda and others discussed a case of a 41-year-old woman with SLE who had recurrent pneumothoraces and was found at autopsy to have blebs in both lungs (81). Others have also described the association of cystic lung disease attributed to SLE with the occurrence of pneumothorax (82). Pulmonary hemorrhage and pulmonary vasculitis have been seen in association with pneumothorax in a patient with SLE (83). Pneumothorax has been associated with nephritis in a patient with SLE (84). Tanaka and colleagues described a 37-year-old woman with SLE complicated by serositis and treated with steroids who developed bilateral spontaneous pneumothoraces (85).
Rheumatoid arthritis
More than 50 years ago, Hindle and Yates described a case of a 40-year-old man with rheumatoid arthritis who had pulmonary nodules which subsequently developed cavities and ruptured leading to a pyopneumothorax (86). A similar case was reported in the following year by Davies (87). Bilateral concurrent pneumothoraces in a patient with nodular lung disease was reported by Burrows (88). Crisp and co-workers reported a small case series of four patients who had lung disease attributed to rheumatoid arthritis, spontaneous pneumothorax, and peripheral eosinophilia (89). Rueth and colleagues have noted that the treatment of spontaneous pneumothorax in patients with rheumatoid arthritis may be complicated by the presence of a bronchopleural fistula (90). In patients with recurrent pneumothorax, Borges and co-workers suggest from their experience with one case that immunosuppressive agents may need to be discontinued in order to allow pleural symphysis to occur (91). Bilateral and recurrent pneumothoraces have been described in patients with rheumatoid arthritis in association with treatment with potent immunomodulating agents (92,93). Pleural disease and pneumothorax associated with mycobacterium avium infection has been reported in a patient with rheumatoid arthritis treated with a tumor necrosis factor (TNF)-alpha antagonist (94).
Scleroderma
Several early reports describe isolated cases of the occurrence of spontaneous pneumothorax in association with scleroderma (95-97). Ng and Tan reported two cases of bilateral spontaneous pneumothorax associated with scleroderma (98). It has recently been reported that spontaneous pneumothorax may be the first manifestation of scleroderma (99,100).
Dermatomyositis
Pneumothorax and pneumomediastinum have rarely been reported in children and adults with dermatomyositis (101-104).
Inflammatory bowel disease
Smith and colleagues recently reported that a patient with Crohn’s disease suffered a spontaneous pneumothorax (105). During video-assisted thoracoscopy, blebs were identified on the surface of the lungs. Histologic section of these lesions demonstrated granulomatous inflammation. Patients with inflammatory bowel disease have been reported to have pneumothorax as a complication of vomiting and invasive procedures (106,107).
Birt-Hogg-Dubé syndrome
Birt-Hogg-Dubé syndrome (BHD) is a rare genetic disorder associated with spontaneous pneumothorax (108-111). BHD is an autosomal dominant condition associated with a gene mapped to chromosome 17p11.2 which expresses the protein folliculin (108). This condition is associated with benign tumors of hair follicles, renal tumors, and spontaneous pneumothorax (108-124). The odds ratio for the development of spontaneous pneumothorax in a patient with BHD has been calculated to be 50% (109). Patients with BHD are predisposed to the development of parenchymal lung cysts (110-124). The development of parenchymal lung cysts in BHD is closely associated with the occurrence of spontaneous pneumothorax (110).
Summary
Spontaneous pneumothorax may be associated with rare diseases such as LAM and PLCH, or may be the rare sequella of more common systemic diseases. Spontaneous pneumothorax is often associated in these cases with parenchymal pulmonary disease, which may predispose patients to recurrent pneumothorax and bilateral pneumothorax. The consensus of most experts is that the first episode of pneumothorax in patients with these underlying conditions be followed by aggressive preventative measures to avoid future occurrences (30).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- McCormack FX, Sullivan EJ, Inoue Y. Lymphangioleiomyomatosis. In: Mason RJ, Broaddus C, Martin T, et al, eds. Murray and Nadel’s Textbook of Respiratory Medicine. Elsevier, 2013.
- McCormack FX. Lymphangioleiomyomatosis: A clinical update. Chest 2008;133:507-16. [PubMed]
- Meraj R, Wilkenheiser-Brokamp KA, Young LR, et al. Lymphangioleiomyomatosis: New concepts in pathogenesis, diagnosis, and treatment. Semin Respir Crit Care Med 2012;33:486-97. [PubMed]
- Crooks DM, Pacheco-Rodriguez G, DeCastro RM, et al. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci USA 2004;101:17462-7. [PubMed]
- Gomez M, Sampson J, Whittemore V. eds. The Tuberouos Sclerosis Complex, 3rd ed. Oxford, England: Oxford University Press, 1999.
- Lautenbacher R. Dysembryomes metotipiques des reins, carcinose submilière aigue poumon avec emphysème généralisé et double pneumothorax. Ann Med Interne 1918;5:435-50.
- Almoosa KF, Ryu JH, Mendez J, et al. Management of pneumothorax in lymphangioleiomyomatosis: effect on recurrence and lung transplantation complications. Chest 2006;129:1274-81. [PubMed]
- Urban T, Lazor R, Lacronique J, et al. Pulmonary lymphangioleiomyomatosis: A study of 69 patients. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM”O”P). Medicine 1999;78:321-37. [PubMed]
- Matsumoto Y, Horiba K, Usuki J, et al. Markers of cell proliferatin and expression of melanosomal antigen in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol 1999;21:327-36. [PubMed]
- Berger U, Khaghani A, Pomerance A, et al. Pulmonary lympangioleiomyomatosis and steroid receptors. An immunocytochemical study. Am J Clin Pathol 1990;93:609-14. [PubMed]
- Almoosa KF, McCormack FX, Sahn SA. Pleural disease in lymphangioleiomyomatosis. Clin Chest Med 2006;27:355-68. [PubMed]
- Kitaichi M, Nishimura K, Itoh H, et al. Pulmonary lymphangioleiomyomatosis: A report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med 1995;151:527-33. [PubMed]
- van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1on chromosome 9q34. Science 1997;277:805-8. [PubMed]
- European Chromosome 16 Tuberous Sclerosis Consortium: Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 1993;75:1305-15. [PubMed]
- Astrinidis A, Cash TP, Hunter DS, et al. Tuberin, the tuberous sclerosis complex 2 tumor suppressor gene product, regulates Rho activation, cell adhesion, and migration. Oncogene 2002;21:8470-6. [PubMed]
- Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary Lymphangioleiomyomatosis. Proc Natl Acad Sci USA 2000;97:6085-90. [PubMed]
- O’Brien JD, Lium JH, Parosa JF, et al. Lymphangioleiomyomatosis recurrence in the allograft after single lung transplantation. Am J Respir Crit Care Med 1995;151:2033-6. [PubMed]
- Bittmann I, Rolf B, Amann G, et al. Recurrence of lymphangioleiomyomatosis after single lung transplantation: New insights into pathogenesis. Hum Pathol 2003;34:95-8. [PubMed]
- Corrin B, Liebow AA, Friedman PJ. Pulmonary Lymphangioleiomyomatosis: a review. Am J Pathol 1975;79:348-82. [PubMed]
- Taylor JR, Ryu J, Colby TV, et al. Lymphangioleiomyomatosis: Clinical course in 32 patients. N Engl J Med 1990;323:1254-60. [PubMed]
- Chu SC, Horiba K, Usuki J, et al. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest 1999;115:1041-52. [PubMed]
- Oh Y-M, Mo EK, Jang SH, et al. Pulmonary lymphangioleiomyomatosis in Korea. Thorax 1999;54:618-21. [PubMed]
- Johnson SR, Tattersfield AE. Clinical experience of Lymphangioleiomyomatosis in the UK. Thorax 2000;55:1052-7. [PubMed]
- Ryu JH, Beck GJ, Lee JC, et al. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med 2006;173:105-11. [PubMed]
- Cheng YL, Lin YY, Chu SJ, et al. Bilateral spontaneous tension pneumothorax secondary to lymphangioleiomyomatosis. J Trauma 2011;70:E99. [PubMed]
- Khajotia R, Raman S. Bilateral spontaneous persistent open pneumothorax with chylothorax. Can Fam Physician 2012;58:757-60. [PubMed]
- Johnston CR, O’Donnell ME, Ahmed WA, et al. Bilateral pneumothorax in pregnancy unmasking Lymphangioleiomyomatosis. Irish J Med Sci 2011;180:933-4. [PubMed]
- Wu TC, Lai YF, Chao TY, et al. Pulmonary lymphangioleiomyomatosis in a 29 year old woman with bilateral spontaneous pneumothorax: a case report. Chang Gung Med J 2000;23:164-8. [PubMed]
- Berkman N, Bloom A, Cohen P, et al. Bilateral spontaneous pneumothorax as the presenting feature in lymphangioleiomyomatosis. Respir Med 1995;89:381-3. [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [PubMed]
- Young LR, Almoosa KF, Pouock-Barziv S, et al. Patient perspectives on management of pneumothorax in Lymphangioleiomyomatosis. Chest 2006;129:1267-73. [PubMed]
- Pollock-BarZiv S, Cohen MM, Downey GP, et al. Air travel in women with lymphangioleiomyomatosis. Thorax 2007;62:176-80. [PubMed]
- Taveira-DaSilva AM, Burstein D, Hathaway OM, et al. Pneumothorax after air travel in lymphangioleiomyomatosis, idiopathic pulmonary fibrosis, and sarcoidosis. Chest 2009;136:665-70. [PubMed]
- Cohen MM, Freyer AM, Johnson SR. Pregnancy experiences among women with lymphangioleiomyomatosis. Respir Med 2009;103:766-72. [PubMed]
- Taveira-DaSilva AM, Stylianou MP, Hedin CJ, et al. Decline in lung function in patients with lymphangioleiomyomatosis treated with and without progesterone. Chest 2004;126:1867-74. [PubMed]
- Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. The WHO committee on histiocytic/reticulum cell proliferations. Reclassification working group of the Histiocyte Society. Med Pediatr Oncol 1997;29:157-66. [PubMed]
- Vassallo R, Ryu JH, Schroeder DR, et al. Clinical outcomes of pulmonary Langerhans’-cell histiocytosis in adults. N Engl J Med 2002;346:484-90. [PubMed]
- Aricò M, Girschikofsky M, Généreau T, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer 2003;39:2341-8. [PubMed]
- Gaensler EA, Carrington CB. Open biopsy for chronic diffuse infiltrative lung disease: clinical roentgenographic and physiological correlations in 502 patients. Ann Thorac Surg 1980;30:411-26. [PubMed]
- Vassallo R, Ryu JH. Pulmonary Langerhans’ cell histiocytosis. Clin Chest Med 2004;25:561-71. [PubMed]
- Vassallo R, Ryu JH. Smoking-related interstitial lung diseases. Clin Chest Med 2012;33:165-78. [PubMed]
- Zeid NA, Muller HK. Tobacco smoke induced granulomas and tumors: association with pulmonary Langerhans’ cells. Pathology 1995;27:247-54. [PubMed]
- Yousem SA, Colby TV, Chen YY, et al. Pulmonary Langerhan’s cell histiocytosis: molecular analysis of clonality. Am J Surg Pathol 2001;25:630-6. [PubMed]
- Bernstrand C, Cederlund K, Ashtrom L, et al. Smoking preceded pulmonary involvement in adults with Langerhans’ cell histiocytosis diagnosed in childhood. Acta Paediatr 2000;89:1389-92. [PubMed]
- Casolaro MA, Bernaudin JF, Saltini C, et al. Accumulation of Langerhans’ cells on the epithelial surface of the lower respiratory tract in normal subjects in association with cigarette smoking. Am Rev Respir Dis 1988;137:406-11. [PubMed]
- Vassallo R, Jensen EA, Colby TV, et al. The overlap between respiratory bronchiolitis and desquamative interstitial pneumonia in pulmonary Langerhans’ cell histiocytosis: high resolution CT, histologic, and functional correlations. Chest 2003;124:1199-205. [PubMed]
- Etienne B, Bertocchi M, Gamondes JP, et al. Relapsing pulmonary Langerhans’ cell histiocytosis after lung transplantation. Am J Respir Crit Care Med 1998;157:288-91. [PubMed]
- Mogulkoc N, Veral A, Bishop PW, et al. Pulmonary Langerhans’ cell histiocytosis: radiologic resolution following smoking cessation. Chest 1999;115:1452-55. [PubMed]
- Hirsch MS, Hong CK. Familial pulmonary histiocytosis-X. Am Rev Respir Dis 1973;107:831-5. [PubMed]
- Travis WD, Borok Z, Roum JH, et al. Pulmonary Langerhans’ cell granulomatosis (histiocytosis X). A clinicopathologic study of 48 cases. Am J Surg Pathol 1993;17:971-86. [PubMed]
- Colby TV, Lombard C. Histiocytosis X in the lung. Hum Pathol 1983;14:847-56. [PubMed]
- Suri HS, Eunhee SY, Nowakowski GS, et al. Pulmonary langerhans cell histiocytosis. Orphanet J Rare Dis 2012;7:16. [PubMed]
- Webber D, Tron V, Askin F, et al. S-100 staining in the diagnosis of eosinophilic granuloma of lung. Am J Clin Pathol 1985;84:447-53. [PubMed]
- Colasante A, Poletti V, Rosini S, et al. Langerhans’ cells in Langerhans’ cell histiocytosis and peripheral adenocarcinomas of the lung. Am Rev Respir Dis 1993;148:752-9. [PubMed]
- Lacronique J, Roth C, Battesti JP, et al. Chest radiological features of pulmonary histiocytosis X: a report based on 50 adult cases. Thorax 1982;37:104-9. [PubMed]
- Friedman PJ, Liebow AA, Sokoloff J. Eosinophilic Granuloma of the lung: clinical aspects of primary histiocytosis in the adult. Medicine 1981;60:385-96. [PubMed]
- Webb WR. High-resolution computed tomography of obstructive lung disease. Radiol Clin North Am 1994;32:745-57. [PubMed]
- Kulwiec EL, Lynch DA, Aguayo SM, et al. Imaging of pulmonary histiocytosis X. Radiographics 1992;12:515-26. [PubMed]
- Ryu JH, Swenson SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc 2003;78:744-52. [PubMed]
- Brauner MW, Grenier P, Mouelhi MM, et al. Pulmonary Langerhans’ cell histiocytosis: evolution of lesions on CT scans. Radiology 1997;204:497-502. [PubMed]
- Buytendijk HJ, Maesen F. Recurrent bilateral pneumothorax in a case of histiocytosis without bone changes. Presse Medicale 1963;71:1300-2. [PubMed]
- Tazi A, Soler P, Hance AJ. Adult pulmonary Langerhans’ cell histiocytosis. Thorax 2000;55:405-16. [PubMed]
- Mendez JL, Nadrous HF, Vassallo R, et al. Pneumothorax in pulmonary Langerhans’ cell histiocytosis. Chest 2004;125:1028-32. [PubMed]
- Minghini A, Trogdon SD. Recurrent spontaneous pneumothorax in pulmonary histiocytosis X. Am Surg 1998;64:1040-2. [PubMed]
- Basset F, Corrin B, Spencer H, et al. Pulmonary histiocytosis X. Am Rev Respir Dis 1978;118:811-20. [PubMed]
- Crausman RS, Jennings CA, Tuder RM, et al. Pulmonary histiocytosis X: pulmonary function and exercise physiology. Am J Respir Crit Care Med 1996;153:426-35. [PubMed]
- Delobbe A, Durieu J, Duhamel A, et al. Determinants of survival in pulmonary Langerhans’ cell granulomatosis (histiocytosis X). Eur Respir J 1996;9:2002-6. [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [PubMed]
- Belhassen-Garcia M, Velasco-Tirado V, Alvela-Suarez L, et al. Spontaneous pneumothorax in Wegener’s Granulomatosis: Case report and literature review. Semin Arthritis Rheum 2011;41:455-60. [PubMed]
- Sezer I, Kocabas H, Melikoglu MA. Spontaneoous pneumothorax in Wegener’s granulomatosis: a case report. Mod Rheumatol 2008;18:76-80. [PubMed]
- Storelli E, Casali C, Natali P, et al. Unusual pathogenesis of spontaneous pneumothorax secondary to Wegener’s granulomatosis. Ann Thorac Surg 2007;84:288-90. [PubMed]
- Delèvaux I, Khellaf M, André M, et al. Spontaneous pneumothorax in Wegener granulomatosis. Chest 2005;128:3074-5. [PubMed]
- Bülbül Y, Ozlü T, Oztuna F. Wegener’s granulomatosis with parotid gland involvement and pneumothorax. Med Princ Pract 2003;12:133-7. [PubMed]
- Michel J, Courthaliac C, Andre M, et al. Quid? Pneumothorax complicating Wegener disease with rupture of pleura of cavitary nodule. Journal de Radiologie 2001;82:73-5. [PubMed]
- Ogawa M, Azemoto R, Makino Y, et al. Pneumothorax in a patient with Wegener’s granulomatosis during treatment with immunosuppressive agents. Journal of Internal Medicine 1991;229:189-92. [PubMed]
- Wolffenbuttel BH, Weber RF, Kho GS. Pyopneumothorax: a rare complication of Wegener’s granulomatosis. Eur J Respir Dis 1985;67:223-7. [PubMed]
- Jaspan T, Davison AM, Walker WC. Spontaneous pneumothorax in Wegener’s granulomatosis. Thorax 1982;37:774-5. [PubMed]
- Epstein DM, Gefter WB, Miller WT, et al. Spontaneous pneumothorax: an uncommon manifestation of Wegener granulomatosis. Radiology 1980;135:327-8. [PubMed]
- Sawkar LA, Easom HF. Recurrent spontaneous pneumothoraces in systemic lupus erythematosus. Chest 1971;60:604-5. [PubMed]
- Richards AJ, Swinson DR, Talbot IC, et al. Diffuse pulmonary fibrosis and bilateral pneumothoraces in systemic lupus erythematosis. Postgrad Med J 1975;51:851-5. [PubMed]
- Masuda A, Tsushima T, Shizume K, et al. Recurrent pneumothoraces and mediastinal emphysema in systemic lupus erythematosus. J Rheumatol 1990;17:544-8. [PubMed]
- Maeda R, Isowa N, Miura H, et al. Systemic lupus erythematosus with multiple lung cysts. Interact Cardiovasc Thorac Surg 2009;8:701-2. [PubMed]
- Nishitsuji M, Nakamura H, Saito K, et al. A case of systemic lupus erythematodes with hemosputum and pneumothorax probably resulting from pulmonary infarction and pulmonary angitis. Nihon Kokyuki Gakkai Zasshi 1998;36:71-6. [PubMed]
- Wilhelm M, Van Why SK. Pneumothoraces complicating systemic lupus erythematosus with nephritis. Pediatr Nephrol 2002;17:261-3. [PubMed]
- Tanaka N, Kusunoki Y, Kaneko K, et al. Systemic lupus erythematosus complicated by recurrent pneumothorax: Case report and literature review. Nihon Rinsho Meneki Gakkai Kaishi 2010;33:162-8. [PubMed]
- Hindle W, Yates DA. Pyopneumothorax complicating rheumatoid lung disease. Ann Rheum Dis 1965;24:57-60. [PubMed]
- Davies D. Pyopneumothorax in rheumatoid lung disease. Thorax 1966;21:230-5. [PubMed]
- Burrows FG. Pulmonary nodules in rheumatoid disease: a report of two cases. Br J Radiol 1967;40:256-61. [PubMed]
- Crisp AJ, Armstrong RD, Grahame R, et al. Rheumatoid lung disease, pneumothorax, and eosinophilia. Ann Rheum Dis 1982;41:137-40. [PubMed]
- Rueth N, Andrade R, Groth S, et al. Pleuropulmonary complications of rheumatoid arthritis: a thoracic surgeon’s challenge. Ann Thorac Surg 2009;88:e20-1. [PubMed]
- Borges H, Schnyder JM, Frey JG, et al. Referring to an unusual case: pulmonary affection and rheumatoid arthritis. Rev Med Suisse 2009;5:2276-80. [PubMed]
- N’Gabou D, Magdeleinat P, Weber N, et al. Pleuropulmonary involvement leading to bilateral pneumothorax in a patient being treated for rheumatoid arthritis. Rev Mal Respir 2010;27:1119-23. [PubMed]
- Kim SH, Yoo WH. Recurrent pneumothorax associated with pulmonary nodules after leflunomide therapy in rheumatoid arthritis: a case report and review of the literature. Rheumatol Int 2011;31:919-22. [PubMed]
- Shimizu T, Ujita M, Numata T, et al. A case of Mycobacterium avium pleuritis and pneumothorax in a rheumatoid arthritis patient treated with a TNF-alpha antagonist. Nihon Kokyuki Gakkai Zasshi 2011;49:583-7. [PubMed]
- Israel MS, Harley BJ. Spontaneous pneumothorax in scleroderma. Thorax 1956;11:113. [PubMed]
- Hyde L. Spontaneous pneumothorax. Dis Chest 1963;43:476-80. [PubMed]
- Edwards WG Jr, Dines DE. Recurrent spontaneous pneumothorax in diffuse scleroderma. Report of a case. Dis Chest 1966;49:96-8. [PubMed]
- Ng SC, Tan WC. Bilateral spontaneous pneumothorax in systemic sclerosis--report of two cases. J Rheumatol 1990;17:689-91. [PubMed]
- Zeuner M, Müller-Ladner U, Mohr VD, et al. Spontaneous pneumothorax in a patient with systemic sclerosis. Clin Rheumatol 1996;15:211-3. [PubMed]
- Watanabe S, Tambo Y, Waseda Y, et al. Pneumothorax as a first manifestation of SS. Rheumatology 2012;51:1334-6. [PubMed]
- Singsen BH, Tedford JC, Platzker AC, et al. Spontaneous pneumothorax: a complication of juvenile dermatomyositis. J Pediatr 1978;92:771-4. [PubMed]
- Gayraud M, Lhote F, Valeyre D, et al. Pneumothorax and pneumomediastinum associated with dermatomyositis. Ann Med Interne (Paris) 1989;140:490-1. [PubMed]
- Jansen TL, Barrera P, van Engelen BG, et al. Dermatomyositis with subclinical myositis and spontaneous pneumomediastinum with pneumothorax: case report and review of the literature. Clin Exp Rheumatol 1998;16:733-5. [PubMed]
- Jang KA, Kim SH, Choi JH, et al. Subcutaneous emphysema with spontaneous pneumomediastinum and pneumothorax in adult dermatomyositis. J Dermatol 1999;26:125-7. [PubMed]
- Smith PA, Crampton JR, Pritchard S, et al. Pneumothorax as a presenting feature of granulomatous disease of the lung in a patient with Crohn’s disease. Eur J Gastroenterol Hepatol 2009;21:237-40. [PubMed]
- Irefin SA, Farid IS, Senagore AJ. Urgent colectomy in a patient with membranous tracheal disruption after severe vomiting. Anesth Analg 2000;91:1300-2. [PubMed]
- Bouma G, van Bodegraven AA, van Waesberghe JH, et al. Post-colonoscopy massive air leakage with full body involvement: an impressive complication with uneventful recovery. Am J Gastroenterol 2009;104:1330-2. [PubMed]
- Khoo SK, Giraud S, Kahnoski K, et al. Clinical and genetic studies of Birt-Hogg-Dubé syndrome. J Med Genet 2002;39:906. [PubMed]
- Zbar B, Alvord WG, Glenn G, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in Bit-Hogg-Dubé syndrome. Cancer Epidemiol Biomarkers Prev 2002;11:393. [PubMed]
- Toro JR, Pautler SE, Stewart L, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg Dubé syndrome. Am J Respir Crit Care Med 2007;175:1044. [PubMed]
- Agarwal PP, Gross BH, Holloway BJ, et al. Thoracic CT findings in Birt-Hogg-Dube syndrome. AJR Am J Roentgenol 2011;196:349-52. [PubMed]
- Tsakiridis K, Mpakas A, Kesisis G, et al. Lung inflammatory response syndrome after cardiac-operations and treatment of lornoxicam. J Thorac Dis 2014;6:S78-98. [PubMed]
- Tsakiridis K, Zarogoulidis P, Vretzkakis G, et al. Effect of lornoxicam in lung inflammatory response syndrome after operations for cardiac surgery with cardiopulmonary bypass. J Thorac Dis 2014;6:S7-20. [PubMed]
- Argiriou M, Kolokotron SM, Sakellaridis T, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis 2014;6:S52-9. [PubMed]
- Madesis A, Tsakiridis K, Zarogoulidis P, et al. Review of mitral valve insufficiency: repair or replacement. J Thorac Dis 2014;6:S39-51. [PubMed]
- Siminelakis S, Kakourou A, Batistatou A, et al. Thirteen years follow-up of heart myxoma operated patients: what is the appropriate surgical technique? J Thorac Dis 2014;6:S32-8. [PubMed]
- Foroulis CN, Kleontas A, Karatzopoulos A, et al. Early reoperation performed for the management of complications in patients undergoing general thoracic surgical procedures. J Thorac Dis 2014;6:S21-31. [PubMed]
- Nikolaos P, Vasilios L, Efstratios K, et al. Therapeutic modalities for Pancoast tumors. J Thorac Dis. 2014;6:S180-93. [PubMed]
- Koutentakis M, Siminelakis S, Korantzopoulos P, et al. Surgical management of cardiac implantable electronic device infections. J Thorac Dis 2014;6:S173-9. [PubMed]
- Spyratos D, Zarogoulidis P, Porpodis K, et al. Preoperative evaluation for lung cancer resection. J Thorac Dis 2014;6:S162-6. [PubMed]
- Porpodis K, Zarogoulidis P, Spyratos D, et al. Pneumothorax and asthma. J Thorac Dis. 2014;6:S152-61. [PubMed]
- Panagopoulos N, Leivaditis V, Koletsis E, et al. Pancoast tumors: characteristics and preoperative assessment. J Thorac Dis 2014;6:S108-15. [PubMed]
- Visouli AN, Darwiche K, Mpakas A, et al. Catamenial pneumothorax: a rare entity? Report of 5 cases and review of the literature. J Thorac Dis 2012;4:17-31. [PubMed]
- Zarogoulidis P, Chatzaki E, Hohenforst-Schmidt W, et al. Management of malignant pleural effusion by suicide gene therapy in advanced stage lung cancer: a case series and literature review. Cancer Gene Ther 2012;19:593-600. [PubMed]