Experimentation with aerosol bonsetan, pirfenidone, treprostinil and sidenafil
Introduction
Inhaled therapies have been used for respiratory diseases and symptom relief since many years and vast experience exists for many products (1-4). Several drugs are also being investigated whether they could be administered as aerosol (5-9). The major obstacle for efficient aerosol deposition are the defense mechanisms of the respiratory system (10) and the drug design for efficient tissue distribution (11). Moreover; safety concerns for novel methods of drugs as aerosol administration, still remain to be investigated and elicited (8,12-16). Previous research studies have identified several parameters that influence the droplet size production and can be summarized to the following: (I) residual cup loading; (II) initial loading of the residual cup; (III) refilling of the residual cup when the initial filling has been reduced to half (this can be done only once); (IV) residual cup design; (V) inlet design (if used) and (VI) drug formulation (17-20). Currently there is an increasing interest in the investigation for drugs administered as aerosol for pulmonary hypertension (PH) and idiopathic pulmonary fibrosis (IPF). PH is defined as a mean pulmonary arterial pressure (PAP) ≥25 mmHg at rest when assessed with right heart catheterization (21). It is a fatal disease caused by vascular proliferation, remodeling and small vessel obstruction. Increased pulmonary vascular resistance (PVR) which is often observed, leads to right-sided heart failure (HF) and death (22). Current guidelines have divided PH in five major categories (23). Currently there are new insights presented for the pathophysiology of the disease and progress in the diagnosis. The diagnosis includes (I) right-heart catheterization; (II) optical coherence tomography and (III) computed tomography. Current treatment strategies for pre-capillary PAH include: (I) lifestyle modification; (II) endothelin receptor antagonists; (III) prostacyclins and (IV) immunosuppressive therapy. Novel agents currently considered are: riociquat, imatinib and Rho-kinase inhibitors. Novel agents for post-capillary PAH include: sildenafil, riociquat and Rho-kinase inhibitors. Previous drugs include: angiotensin-receptor blockers, nitrates, diuretics, angiotensin-converting enzyme inhibitors and β-blockers. Pulmonary artery angioplasty is considered the treatment for chronic thromboembolic PH (24). Currently inhaled treprostinil (Tyvaso) has been approved by the FDA [2009] for patients with PAH with NYHA III. Prostcyclin-2 through cycloxygenase-2, leads to vasodilation and platelet aggregation (25). Prostacyclin causes vasodilation in both systemic and pulmonary arteries. Moreover; it has antiproliferative activity as observed with the inhibition of growth of smooth muscle cells. Inhaled prostanoids are usually administered in patients who are not candidates for parenteral treatment, or with declining condition. The major advantage is the delivery of the drug directly to the lungs and therefore less systemic side effects have been observed (4,26,27). Inhaled treprostinil through the TRIUMPH trial provided data where the 6-minute walking test was improved when compared to stable doses of bonsentan or sildenafil (28). IPF is a debilitating disease occurring in adults between 60 to 75 years of age (29). The disease is fatal with a median survival of 2-5 years, and progressive pulmonary function occurs. Pirfenidone is indicated for mild to moderate IPF based on phase III trials using forced vital capacity (FVC) ≥50%, carbon monoxide diffusing capacity (DLCO) ≥35% and 6-minute walking test (6MWT) distance of ≥150 m (30). Additional drugs that have presented positive results as additional treatment for IPF are N-acetylcysteine (NAC) and nintedanib (BIBF 1120), however; further investigation is necessary (31,32). In the current study we have investigated whether modification of the drugs bonsentan, sildenafil, treprostinil and pirfenidone with different nebulizers, residual cups and loadings could be used as a future local administration to the lung parenchyma.
Materials and methods
Drugs
The following drugs were purchased: (I) Tracleer® (bonsetan) 62.5 mg/tab, Actelion; (II) Revatio®, (sildenafil) 20 mg/tab, Pfizer; (III) Esbriet® (pirfenidone) 276 mg/hard capsule, Intermune UK Ltd.; and (IV) Remodulin® (treprostinil) 5 mg/mL solution, United Therapeutics Europe Ltd.
Nebulizers and residual cups
Jet-nebulizers and residual cups
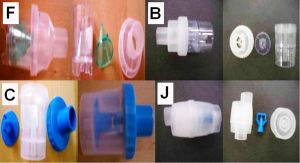
Three nebulizers were chosen from our department for the experiment: Maxineb® (6 liters/minute and 35 psi), Sunmist® (5-7 liters/minute and 35 psi) and Invacare® (4-8 liters-minute and 36 psi). In total seven residual cups were chosen for evaluation, four with a capacity of no more than 6 mLs and two with a capacity no more than 10 mLs. The designs for the large residual cups will be mentioned as A, D and E. The small residual cups will be mentioned as C, F, B and J. The large residual cups were not used with a capacity of more than 8 mLs as explained in the discussion section (Figure 1).
Ultrasound nebulizers
Three new ultrasound nebulizers were chosen from the market based on their cost-effectiveness. The first was Omron® NE-U07, Tokyo, Japan. Compact and weight less than 350 gm, includes 10 mL medication cup. Generates uniform micro millimetre-sized vapor particles. The second was a portable GIMA, Gessate, Italy (Choice Smart Health Care Company Limited, Wan Chai, Hong Kong, No. G2061259328002) with the following operating specifications; particle size: 3-5 µm, frequency: 2.5 MHz, medication cup capacity: 1-6 mLs, sound level at 10 cm: <50 db, operating temperature: min. 10 °C, max. 40 °C and air humidity: min. 10%, max. 95% RH. The third was a portable EASYneb® II, FLAEMNUOVA, Martino, Italy. with the following operating specifications; drug max capacity: 8 mLs, frequency: 2.4 MHz, nebulization capacity (adjustable) 0-0.7 mL/min approximately (tests performed with saline 0.9%), particle size: 2.13 µm mass median aerodynamic diameter (MMAD), sound level at 10 cm: 50 db (A), operating temperature: min. 10 °C, max. 40 °C and air humidity: min. 10%, max. 95% RH (Figures 2-4).
Measurement of droplet size and droplet size distribution
A laser scattering apparatus (Malvern Mastersizer 2000, Malvern, Worcestershire, UK) equipped with a Scirocco dry accessory module (Malvern, Worcestershire, UK) was used for the determination of the mass median diameter of the produced particles. A specific surface area odd 4.3 was used. Light scattering was used instead of a cascade impactor, as (I) a cascade impactor is, by design, limited to the number of size populations it may discriminate, that is the number of filters. In such a case, a limited number of populations can be distinguished. Our light scattering set-up can measure the light scattering intensity at a very large number of angles (that is the equivalent of a cascade impactor filters), so it can construct a particle size distribution plot of a very large number of points; (II) light scattering is non-invasive to all particles. The above, coupled with the application of the very accurate ‘Mie theory’ used here for transferring the angle-intensity measurements into size-volume data, and the thorough control of our data fitting, give us confidence in the presented results. Our equipment has been previous used in prior publications (5-8,17) (Figure 5).
Milling
The bonsetan, pirfenidone and sidenafil tablets were milled in a planetary ball mill (Frisch, Pulverisette-5) equipped with Agate bowls (500 mL) and 8 balls (20 mm, 20 g) with a rotational speed of approximately 200 rpm which results in an acceleration of about 7.5 g. We initiated our milling at 40 minutes and we acquired a MMAD of 4.4 µm for bonsetan and 3.7 for pirfenidone. The major problem that we encountered was that the sidenafil tablets were influenced by the heat that was developed during the process and a paste was developed around the walls of the agate bowls (Figure 6). Therefore we had to seek for an alternative method of milling and we used a grinder FALIING NUMBER S-12611, Stockholm, Sweden, type 120, 3-phase and 280 rpm, made in Finland. Before proceeding to milling we had measured the weight of the tablets and capsules with a digital scale Kern EG 2200-2NM, Kern & Sohn, GmbH, Balingen, Germany (Figure 7). After milling we collected powder of the same weight from drug and diluted it with 2 mLs in an effort to simulate a future method/compound of administration.
Statistical assessment
MMAD was measured following three different experimental factorial designs:
- Three jet nebulizers in joint with four drugs, seven cup devices with three dosage levels each (2, 4 and 6 mL) were analyzed for potential effects on droplet size by employing a four-factor ANOVA, fixed effects plus their interaction levels, thus totaling 3×4×7×3=252 combined levels;
- The same design was repeated including only the large residual cups (A, D, E) at 8 mL dose level using a tree-factor ANOVA and totaling 3×4×3=27 combined levels;
- The droplet size was finally checked for potential effects by applying three ultrasound nebulizers, the same drugs as before and “delivery” constructions (face mask and inlet) at two dose levels (2 and 4 mL). A four-factor ANOVA, fixed effects, was performed making up 3×4×3×2=72 combined levels.
Statistically significant effects and interaction terms were tested graphically using pair-wise comparisons of means together with their 95% confidence intervals. Means whose intervals do not overlap are significantly different.
The major aim is focused on the best combination that provides droplets with the least size.
Results
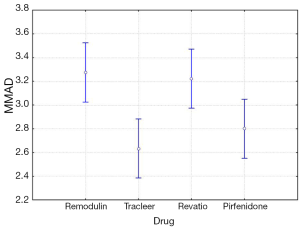
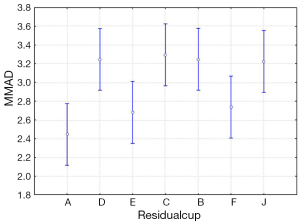
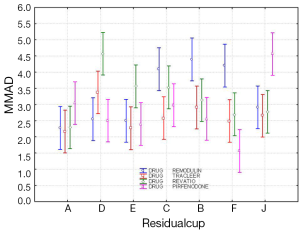
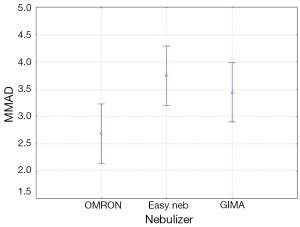
The four-way ANOVA on droplet size using the jet nebulizers revealed two statistically significant factors, drug (F=6.326, P=0.0007) and residual cup (F=4.419, P=0.0007), and their interaction term (F=5.829, P<0.0001). Drugs tracleer and pirfenidone produce equally the lowest mean droplet size (2.63 and 2.80 respectively) as compared to other two drug mean sizes (Figure 8). Two groups of different mean droplet size are produced by the residual cups (Figure 9). Cups A, E and F are equally the most efficient in small mean droplet size (2.44, 2.68 and 2.74 respectively), while the rest four cups form another group with higher mean sizes, ranging between 3.22 and 3.30). Cup F in joint with drug pirfenidone (Figure 10) create the least mean droplet size (1.56), by far lower from all other combined levels and particularly from the combined effect of drug remodulin with cups C, B, and F whose result rises up to 4.1-4.4 µm. The large residual cups (A, D and E), when combined with the four aforesaid drugs and the same nebulizers, do not produce statistically significant results at 8 mL dose level (P>0.05 in all factors and interaction terms). The ANOVA results, concerning the ultrasound nebulizers, revealed only the nebulizers as producing significant effect on droplet size (F=4.753, P=0.037). This effect is easily clarified in the Figure 11 in which the OMRON nebulizer outweighs the others’ effect providing a mean droplet size of 2.69 µm. To capitalize, jet nebulizers, although not significant on droplet size, best perform with drugs tracleer and pirfenidone, using cup designs A, E and F, and particularly when combined with cup F and pirfenidone. Loadings greater than 6 mL together with any other factor combined do not exert any significant effect on droplet size. Ultrasound nebulizers produce a unique effect on droplet size, no matter considering any other factor in the study, from which OMRON generates the smallest droplets.
Discussion
Inhalation therapies in order to be efficient they have to bypass the defense mechanisms of the respiratory system. These can be summarized to: (I) beating cilia; (II) macrophages; (III) mucus; (IV) local enzymes; and (V) local transporters (10). Furthermore; an underlying respiratory disease modifies the deposition and absorption of aerosol therapies. Bronchoconstriction which might occur during an exacerbation of asthma or chronic obstructive pulmonary disease (COPD) reduces the distribution and deposition of an aerosol therapy (33,34). The production of mucus also reduces the absorption of an aerosol therapy since a concentration of the drug is enclosed within the mucus. Local enzymes and transporters are parameters that their interaction with the aerosol drugs has not been fully investigated. It has been presented that there are different enzymes and transporters at different locations of the respiratory system which modify the absorption of a drug locally (35). Moreover, the aerosol mist has to be composed of droplets of MMAD of <5 µm. The third important factor for an efficient aerosol therapy is the drug design. There are two major delivery systems for local absorption; the passive and active transportation. The passive transportation is based on the principals of the drug formulation molecules and interaction with the local enzymes and transporters. The active transportation is based on an antigen-antibody connection of the drug molecules and local tissue (11). Active transportation tends to be usually less expensive in production, however, in several situations less effective. Cost-effective design is another parameter that has to be included in drug design development. Previous investigation of aerosol production systems have indicated that jet-nebulizers and ultrasound nebulizers are easy to use in the everyday practice, however, jet-nebulizers are currently cheaper than ultrasound nebulizers. Moreover, it has been observed that several drugs are more efficiently delivered with one system versus the other, meaning that we should choose the aerosol production system based also on the drug that we want to deliver (20). The production of aerosol regarding the jet-nebulizers is also influenced by the residual cup design, initial filling, time of nebulisation and drug. Moreover; it has been observed that reduction of the produced droplet size can be achieved with the usage of inlets design (17). Temperature increase from the piezoelectric crystal results in a higher concentration effect of the drug solution, than in the jet-nebulizers (36). However, the combination of temperature and drug concentration cause a shift in the tension and viscosity and consequently change the droplet size distribution (37). The nebulization time also plays a role in the viscosity, surface tension, saturated vapour pressure and finally to the droplet size distribution. Increase of the concentration in the produced aerosol is mostly observed in the ultrasound nebulizers than the jet-nebulizers (38). The variations of the viscosity are also time dependent (low the first 2 minutes, higher after the 4 minutes) and temperature dependent in ultrasound nebulizers. An increase in the mean droplet size range has been also observed when adding buffer (39). The increasing drug concentration is also a factor inducing bronchoconstriction (40). In conclusion the major factors affecting the produced mist are: (I) temperature; (II) viscocity; (III) drug formulations (salts, buffer) and (IV) time of nebulization.
PAH is a complex disease characterized by increased PVR which eventually leads to right-sided HF and death (41). Current recommendations for patients with NYHA II, III, IV symptoms indicate first-line monotherapy with agents targeting the following pathways: endothelin, prostacyclin and nitric oxide. Sequential combination of agents is indicated when there is no evidence of clinical improvement with one specific-PAH agent (42). However, again there are patients who do not present improvement even after a combination treatment (43). Combination treatment has been proposed in advanced stage disease for initial treatment (42). The concept for combination treatment has the advantage of targeting simultaneously different pathological pathways (43). Most studies have investigated the sequential addition of agents and very few initiation of a combination treatment (44). Currently pharmacogenomics are investigated for targeted treatment of PAH as a future treatment (45). Possibly in the future we could target the disease with a combination of aerosol treatment. Treatment for IPF is based on the updated recommendations of 2013 (29). Several agents have been presented and discussed such as, corticosteroids, NAC, interferon-gamma-1b, azathriopine, anticoagulant therapy, oxygen therapy, lung transplantation and pirfenidone (46). Combination of treatments has been also been proposed (46-58). Additionally, “weak no” has been indicated for treatment of PAH due to IPF according to the last updated recommendations (29). IPF despite current treatment efforts still remains a fatal disease with a median survival between 2-5 years. Currently we investigated whether inhaled pirfenidone could be redesigned to be delivered as aerosol. Major obstacle for IPF is firstly the safety of aerosol administration, because several drugs tend to induce exacerbation of an underlying disease and secondly the efficiency. Production and deposition of a drug does not necessarily mean that the formulation will be distributed from the site of administration to the blood circulation, in specific in patients with IPF since the parenchyma is destroyed. Further investigation has to be investigated probably in a 3D model of lung parenchyma simulating IPF disease.
Conclusions
In our study we investigated drug solutions close to a possible future treatment and we observed that the dilution as it was done it could be a possible method of administration for all drugs. Large concentrations >8 mLs were not necessary as in our previous studies and it is only matter of how much concentration of the drug we want to deliver. Again it was observed that residual cup design plays an important role in the production of the aerosol and the drug formulation. Certainly we need additional local therapies for PH and IPF, local administration has possible advantages, however, future experiments have to present data for the safety of this administration method to the lung.
Acknowledgements
The authors Paul Zarogoulidis, Konstantinos Zarogoulidis and Georgia Pitsiou would like to thank the Special Product Manager Irini Kefalidou for the provided information regarding treprostinil.
Disclosure: The authors declare no conflict of interest.
References
- Râjnoveanu RM, Antoniu S, Ulmeanu R. Combined long-acting bronchodilator single therapy for COPD. Expert Opin Pharmacother 2014;15:139-42. [PubMed]
- Gregersen TL, Ulrik CS. Safety of bronchodilators and corticosteroids for asthma during pregnancy: what we know and what we need to do better. J Asthma Allergy 2013;6:117-25. [PubMed]
- Poms A, Kingman M. Inhaled treprostinil for the treatment of pulmonary arterial hypertension. Crit Care Nurse 2011;31:e1-10. [PubMed]
- Channick RN, Voswinckel R, Rubin LJ. Inhaled treprostinil: a therapeutic review. Drug Des Devel Ther 2012;6:19-28. [PubMed]
- Zarogoulidis P, Darwiche K, Huang H, et al. Time recall; future concept of chronomodulating chemotherapy for cancer. Curr Pharm Biotechnol 2013;14:632-42. [PubMed]
- Zarogoulidis P, Darwiche K, Krauss L, et al. Inhaled cisplatin deposition and distribution in lymph nodes in stage II lung cancer patients. Future Oncol 2013;9:1307-13. [PubMed]
- Zaric B, Stojsic V, Tepavac A, et al. Adjuvant chemotherapy and radiotherapy in the treatment of non-small cell lung cancer (NSCLC). J Thorac Dis 2013;5 Suppl 4:S371-7. [PubMed]
- Zarogoulidis K, Boutsikou E, Zarogoulidis P, et al. The role of second-line chemotherapy in small cell lung cancer: a retrospective analysis. Onco Targets Ther 2013;6:1493-500. [PubMed]
- Zarogoulidis P, Petridis D, Ritzoulis C, et al. Further experimentation of inhaled; LANTUS, ACTRAPID and HUMULIN with todays’ production systems. Int J Pharm 2013;458:39-47. [PubMed]
- Zarogoulidis P, Darwiche K, Yarmus L, et al. Defense mechanisms of the respiratory system and aerosol production systems. Med Chem 2014;10:123-36. [PubMed]
- Bae YH. Interview with Dr You Han Bae: ligand-mediated versus ‘passive’ targeting approaches in nanoparticle oncology research. Ther Deliv 2012;3:933-6. [PubMed]
- Darwiche K, Zarogoulidis P, Karamanos NK, et al. Efficacy versus safety concerns for aerosol chemotherapy in non-small-cell lung cancer: a future dilemma for micro-oncology. Future Oncol 2013;9:505-25. [PubMed]
- Zarogoulidis P, Giraleli C, Karamanos NK. Inhaled chemotherapy in lung cancer: safety concerns of nanocomplexes delivered. Ther Deliv 2012;3:1021-3. [PubMed]
- Zarogoulidis P, Eleftheriadou E, Sapardanis I, et al. Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. Invest New Drugs 2012;30:1628-40. [PubMed]
- Zarogoulidis P, Kontakiotis T, Zarogoulidis K. Inhaled gene therapy in lung cancer: “as for the future, our task is not to foresee it, but to enable it Ther Deliv 2012;3:919-21. [PubMed]
- Zarogouldis P, Karamanos NK, Porpodis K, et al. Vectors for inhaled gene therapy in lung cancer. Application for nano oncology and safety of bio nanotechnology. Int J Mol Sci 2012;13:10828-62. [PubMed]
- Boukovinas I, Tsakiridis K, Zarogoulidis P, et al. Neo-adjuvant chemotherapy in early stage non-small cell lung cancer. J Thorac Dis 2013;5 Suppl 4:S446-8. [PubMed]
- Ferron GA, Kerrebijn KF, Weber J. Properties of aerosols produced with three nebulizers. Am Rev Respir Dis 1976;114:899-908. [PubMed]
- Kendrick AH, Smith EC, Wilson RS. Selecting and using nebuliser equipment. Thorax 1997;52 Suppl 2:S92-101. [PubMed]
- Laube BL, Janssens HM, de Jongh FH, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J 2011;37:1308-31. [PubMed]
- Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S55-66. [PubMed]
- Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 2004;43:13S-24S. [PubMed]
- Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009;54:S43-54. [PubMed]
- Fukumoto Y, Shimokawa H. Recent progress in the management of pulmonary hypertension. Circ J 2011;75:1801-10. [PubMed]
- Vane J, Corin RE. Prostacyclin: a vascular mediator. Eur J Vasc Endovasc Surg 2003;26:571-8. [PubMed]
- Hohenforst-Schmidt W, Hornig J, Friedel N, et al. Successful management of an inadvertent excessive treprostinil overdose. Drug Des Devel Ther 2013;7:161-5. [PubMed]
- Channick RN, Olschewski H, Seeger W, et al. Safety and efficacy of inhaled treprostinil as add-on therapy to bosentan in pulmonary arterial hypertension. J Am Coll Cardiol 2006;48:1433-7. [PubMed]
- Benza RL, Seeger W, McLaughlin VV, et al. Long-term effects of inhaled treprostinil in patients with pulmonary arterial hypertension: the Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension (TRIUMPH) study open-label extension. J Heart Lung Transplant 2011;30:1327-33. [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [PubMed]
- Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011;377:1760-9. [PubMed]
- Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med 2011;365:1079-87. [PubMed]
- Idiopathic Pulmonary Fibrosis Clinical Research Network, Raghu G, Anstrom KJ, et al. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 2012;366:1968-77. [PubMed]
- Barnes N, Calverley PM, Kaplan A, et al. Chronic obstructive pulmonary disease and exacerbations: clinician insights from the global Hidden Depths of COPD survey. Curr Med Res Opin 2014;30:667-84. [PubMed]
- Patel M, Pilcher J, Pritchard A, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerbations: a randomised controlled trial. Lancet Respir Med 2013;1:32-42. [PubMed]
- Bosquillon C. Drug transporters in the lung--do they play a role in the biopharmaceutics of inhaled drugs? J Pharm Sci 2010;99:2240-55. [PubMed]
- Niven RW, Ip AY, Mittelman S, et al. Some factors associated with the ultrasonic nebulization of proteins. Pharm Res 1995;12:53-9. [PubMed]
- Finlay WH. eds. The mechanisms of inhaled pharmaceutical aerosols. London: Academic Press, 2001.
- O’Callaghan C, Barry PW. The science of nebulised drug delivery. Thorax 1997;52 Suppl 2:S31-44. [PubMed]
- Steckel H, Eskandar F. Factors affecting aerosol performance during nebulization with jet and ultrasonic nebulizers. Eur J Pharm Sci 2003;19:443-55. [PubMed]
- Hickey AJ. eds. Pharmaceutical inhalation aerosol technology. New York: Marcer Decker, 1992.
- Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2004;351:1425-36. [PubMed]
- Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC), European Respiratory Society (ERS), International Society of Heart and Lung Transplantation (ISHLT), et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009;34:1219-63. [PubMed]
- Hoeper MM, Faulenbach C, Golpon H, et al. Combination therapy with bosentan and sildenafil in idiopathic pulmonary arterial hypertension. Eur Respir J 2004;24:1007-10. [PubMed]
- Kemp K, Savale L, O’Callaghan DS, et al. Usefulness of first-line combination therapy with epoprostenol and bosentan in pulmonary arterial hypertension: an observational study. J Heart Lung Transplant 2012;31:150-8. [PubMed]
- Said SI, Hamidi SA. Pharmacogenomics in pulmonary arterial hypertension: Toward a mechanistic, target-based approach to therapy. Pulm Circ 2011;1:383-8. [PubMed]
- Behr J, Richeldi L. Recommendations on treatment for IPF. Respir Res 2013;14 Suppl 1:S6. [PubMed]
- Hohenforst-Schmidt W, Zarogoulidis P, Linsmeier B, et al. Enhancement of aerosol cisplatin chemotherapy with gene therapy expressing abc10 protein in respiratory system. J Cancer 2014;5:344-50. [PubMed]
- Angelis N, Porpodis K, Zarogoulidis P, et al. Airway inflammation in chronic obstructive pulmonary disease. J Thorac Dis 2014;6 Suppl 1:S167-72. [PubMed]
- Papaiwannou A, Zarogoulidis P, Porpodis K, et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis 2014;6 Suppl 1:S146-51. [PubMed]
- Walter RF, Zarogoulidis P, Mairinger FD, et al. Cell viability of fibroblasts to pifenidone and sirolimus: a future concept for drug eluting stents. Int J Pharm 2014;466:38-43. [PubMed]
- Zarogoulidis P, Darwiche K, Tsakiridis K, et al. Learning from the Cardiologists and Developing Eluting Stents Targeting the Mtor Pathway for Pulmonary Application; A Future Concept for Tracheal Stenosis. J Mol Genet Med 2013;7:65. [PubMed]
- Zarogoulidis P, Porpodis K, Kioumis I, et al. Experimentation with inhaled bronchodilators and corticosteroids. Int J Pharm 2014;461:411-8. [PubMed]
- Zarogoulidis P, Kioumis I, Porpodis K, et al. Clinical experimentation with aerosol antibiotics: current and future methods of administration. Drug Des Devel Ther 2013;7:1115-34. [PubMed]
- Zarogoulidis P, Petridis D, Ritzoulis C, et al. Internal mouthpiece designs as a future perspective for enhanced aerosol deposition. Comparative results for aerosol chemotherapy and aerosol antibiotics. Int J Pharm 2013;456:325-31. [PubMed]
- Zarogoulidis P, Darwiche K, Walter R, et al. Research spotlight: sirolimus-coated stents for airway tracheal stenosis: a future 3D model concept with today’s knowledge. Ther Deliv 2013;4:1093-7. [PubMed]
- Zarogoulidis P, Kioumis I, Ritzoulis C, et al. New insights in the production of aerosol antibiotics. Evaluation of the optimal aerosol production system for ampicillin-sulbactam, meropenem, ceftazidime, cefepime and piperacillin-tazobactam. Int J Pharm 2013;455:182-8. [PubMed]
- Zarogoulidis P, Hohenforst-Schmidt W, Darwiche K, et al. 2-diethylaminoethyl-dextran methyl methacrylate copolymer nonviral vector: still a long way toward the safety of aerosol gene therapy. Gene Ther 2013;20:1022-8. [PubMed]
- Zarogoulidis P, Petridis D, Ritzoulis C, et al. Establishing the optimal nebulization system for paclitaxel, docetaxel, cisplatin, carboplatin and gemcitabine: back to drawing the residual cup. Int J Pharm 2013;453:480-7. [PubMed]