Effusion cytology: an effective method for the diagnosis of pulmonary lymphangioleiomyomatosis
Introduction
Lymphangioleiomyomatosis (LAM), a rare progressive lung neoplasm belonging to the family of perivascular epitheloid cell tumors, mainly affects females of childbearing age. It occurs sporadically or in association with tuberous sclerosis complex (TSC) (1-3). LAM is characterized by the neoplastic smooth muscle-like LAM cells and the diffuse cystic destruction of the lung parenchyma (4,5). Patients with LAM usually present progressive dyspnea on exertion, recurrent pneumothorax, chylous effusions and occasional haemoptysis (1,6). According to the recent European Respiratory Society (ERS) guidelines for the diagnosis and management of LAM, a definite diagnosis of LAM can be established by lung biopsy or high-resolution computed tomography (HRCT) combined with typical clinical features (7). In fact, the cytologic diagnosis of LAM for pleural, ascitic or pericardial fluid samples has already proven feasible and dependable (8-12). However, this effective method was not raised in the ERS guidelines. Here we present an additional case of LAM diagnosed by effusion cytology which can help the patients to avoid an invasive biopsy.
Case report
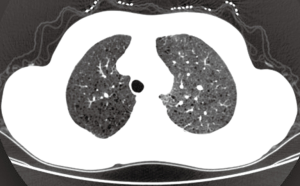
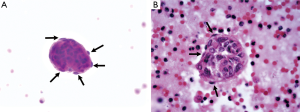
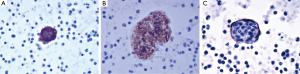
A 39-year-old female nonsmoker was admitted to our hospital because of progressive dyspnea on exertion. A chest CT scan was performed in another hospital where she was treated previously, revealing right pleural effusion. Thereafter, a chest drainage tube was placed. The fluid yielded was milky and processed for cytologic examination. Further chest CT in our hospital showed numerous cystic lesions in bilateral lungs (Figure 1). The cysts are round and thin-walled. Both ThinPrep cytological test (TCT) and cell block analysis for the pleural effusion were performed to detect if there were malignant cells. On TCT slides, some round spool like clusters with a 3-dimensional structure were detected in an inflammatory background interspersed with lymphocytes, granulocytes, macrophages, and some mesothelial cells (Figure 2A). In a close-up view, the clusters included two different kinds of cells (Figure 2A). The inner cells were similar in size and presented a lightly higher nuclear/cytoplasmic ratio, however, mitotic figures were not recognized. The surface of the clusters was composed of flattened cells like lymphatic endothelial cells. Neither tumor diathesis nor solitary LAM cells could be detected on the TCT slides. On the cell block sections, the above clusters were also identified (Figure 2B). To make a better clarification, a panel of immunocytochemistry stainings were performed on cell block sections. The inner cells demonstrated diffuse reactivity for smooth muscle actin (SMA) (Figure 3A) and HMB-45 (Figure 3B). The outer cells were positive for D2-40 (Figure 3C). But the two kinds of cells were negative for Melan-A, estrogen receptor (ER), progestrone receptor (PR), thyroid transcription factor (TTF)-1, AE1/AE3, cytokeratin (CK)7, Calretinin and CK5/6.
Considering the age and gender of the patient, the clinical symptom of dyspnea and chylothorax and the multicystic manifestation of chest CT, a diagnosis of LAM was highly suggested. The cytologic examination of the pleural effusion confirmed what we suspected. An abdomino-pelvic CT revealed there was no evidence of angiomyolipoma and other abdominal lesions.
Discussion
LAM is a rare and slowly progressive systemic disease, and it often causes the ignorance and delay in clinical diagnosis (6,13,14). Based on the study of Taylor et al., the interval between initial symptoms and diagnosis was 44 months (14). It results in the patient losing right clinical treatment including refraining from smoking, inhaled bronchodilators, progesterone and others (7). The ERS guidelines for the diagnosis of LAM were issued in 2010. They emphasize the role of pathological examination based on biopsy, HRCT and clinical manifestation (7). However, cytologic examination based on chylous effusion was not mentioned in the guidelines. Actually, the LAM cell clusters (LCCs) can exist in the chylous effusion has been confirmed by Itami et al. in 1997 (8). What is really exciting is that recognizable LCCs appear to be always detected in chylous effusion, including hydrothorax, ascites and pericardial effusion (12).
A few researchers have previously described the cytologic findings of LAM (8-12,15). The reported cases have common cytologic characters as we described. LCCs, specific feature for LAM, present 3-dimensional spheres on the smear. They are consistently composed of two different cellular components: inner cells and outer cells. The inner cells are oval or spindle, and they have similar size in appearance. The outer layer of cells is scattered, flat and looks like endothelial cells. Histologically, proliferating smooth muscle cells lead to the formation of cysts in pulmonary alveolus and interstitium around the blood vessels and small airways. Chylous effusion might be observed in the thoracic cavity when smooth muscle cells around lymphatic vessels obstructed lymphatic pathways (9,16,17). Immunocytochemical analysis is helpful for confirming the diagnosis of LAM. The inner cells usually show immunoreactivity for SMA and HMB45. In some cases they can also be positive for Melan-A, ER or PR. The superficial cells frequently express D2-40 (10,12,17,18).
In fact, chylous fluid is one of the most frequent complications of LAM (6,13,14). In a large series reported by Chu SC et al., chylothorax was observed in 23% of cases during the course of the disease. Other effusions were ascites (11%) and pericardial effusion (6%) (13). Therefore, in theory, quite a number of LAM cases can be confirmed by cytologic examination. The cell block method was 15% more than the routine conventional smear method in cellularity and additional yield for malignancy (19). In addition, it can be used to perform specific pathology detections, such as molecular testing or immunocytochemistry.
The ERS diagnostic criteria for LAM highlights the function of HRCT but ignores that of conventional CT (7). Characteristic lung HRCT of LAM is multiple (>10) thin-walled round well-defined air-filled cysts throughout the lungs. And the cysts are mostly 2 mm to 2 cm in size (20). However it is difficult to popularize HRCT in every medical center, especially in developing countries. In this case, multiple cysts can also be seen clearly in both lung fields on a conventional chest CT. We believe conventional chest CT can still provide some useful information for pulmonary LAM, although its resolution is lower.
In conclusion, the diagnosis of LAM can be confirmed by cytologic examination based on chylous effusion in conjunction with conventional chest CT and clinical manifestations. This effective method can help some patients to avoid an invasive biopsy.
Acknowledgements
This work was supported by the grant from Science & Technology Development Foundation of Qingdao City (No. 08-2-1-4-nsh).
Disclosure: The authors declare no conflict of interest.
References
- Johnson S. Rare diseases. 1. Lymphangioleiomyomatosis: clinical features, management and basic mechanisms. Thorax 1999;54:254-64. [PubMed]
- Franz DN, Brody A, Meyer C, et al. Mutational and radiographic analysis of pulmonary disease consistent with lymphangioleiomyomatosis and micronodular pneumocyte hyperplasia in women with tuberous sclerosis. Am J Respir Crit Care Med 2001;164:661-8. [PubMed]
- Mavroudi M, Zarogoulidis P, Katsikogiannis N, et al. Lymphangioleiomyomatosis: current and future. J Thorac Dis 2013;5:74-9. [PubMed]
- Meraj R, Wikenheiser-Brokamp KA, Young LR, et al. Lymphangioleiomyomatosis: new concepts in pathogenesis, diagnosis, and treatment. Semin Respir Crit Care Med 2012;33:486-97. [PubMed]
- Avila NA, Chen CC, Chu SC, et al. Pulmonary lymphangioleiomyomatosis: correlation of ventilation-perfusion scintigraphy, chest radiography, and CT with pulmonary function tests. Radiology 2000;214:441-6. [PubMed]
- Urban T, Lazor R, Lacronique J, et al. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM“O”P). Medicine (Baltimore) 1999;78:321-37. [PubMed]
- Johnson SR, Cordier JF, Lazor R, et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 2010;35:14-26. [PubMed]
- Itami M, Teshima S, Asakuma Y, et al. Pulmonary lymphangiomyomatosis diagnosed by effusion cytology. A case report. Acta Cytol 1997;41:522-8. [PubMed]
- Yamauchi M, Nakahara H, Uyama K, et al. Cytologic finding of chyloascites in lymphangioleiomyomatosis. A case report. Acta Cytol 2000;44:1081-4. [PubMed]
- Hirama M, Atsuta R, Mitani K, et al. Lymphangioleiomyomatosis diagnosed by immunocytochemical and genetic analysis of lymphangioleiomyomatosis cell clusters found in chylous pleural effusion. Intern Med 2007;46:1593-6. [PubMed]
- Tynski Z, Eisenberg R. Cytologic findings of lymphangioleiomyomatosis in pleural effusion: a case report. Acta Cytol 2007;51:578-80. [PubMed]
- Mitani K, Kumasaka T, Takemura H, et al. Cytologic, immunocytochemical and ultrastructural characterization of lymphangioleiomyomatosis cell clusters in chylous effusions of patients with lymphangioleiomyomatosis. Acta Cytol 2009;53:402-9. [PubMed]
- Chu SC, Horiba K, Usuki J, et al. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest 1999;115:1041-52. [PubMed]
- Taylor JR, Ryu J, Colby TV, et al. Lymphangioleiomyomatosis. Clinical course in 32 patients. N Engl J Med 1990;323:1254-60. [PubMed]
- Ackley CD, Heineman L, Dodd LG. Utility of fine-needle aspiration in the diagnosis of recurrent pulmonary lymphangioleiomyomatosis: a case report. Diagn Cytopathol 1998;19:458-61. [PubMed]
- Tawfik O, Austenfeld M, Persons D. Multicentric renal angiomyolipoma associated with pulmonary lymphangioleiomyomatosis: case report, with histologic, immunohistochemical, and DNA content analyses. Urology 1996;48:476-80. [PubMed]
- Grzegorek I, Drozdz K, Podhorska-Okolow M, et al. LAM cells biology and lymphangioleiomyomatosis. Folia Histochem Cytobiol 2013;51:1-10. [PubMed]
- Lu SH, Hou YY, Tan YS, et al. Clinical and histopathological alterations of lymphangioleiomyomatosis in 14 Chinese patients. Chin Med J (Engl) 2009;122:1895-900. [PubMed]
- Shivakumarswamy U, Arakeri SU, Karigowdar MH, et al. Diagnostic utility of the cell block method versus the conventional smear study in pleural fluid cytology. J Cytol 2012;29:11-5. [PubMed]
- Ryu JH, Tian X, Baqir M, et al. Diffuse cystic lung diseases. Front Med 2013;7:316-27. [PubMed]