Infrequent ERBB2 mutations in Chinese patients with non-small cell lung cancer
Introduction
Members of c-erb B family of oncogenes, which include epidermal growth factor receptor (EGFR, ERBB1), ERBB2 (HER2), ERBB3 (HER3), and ERBB4 (HER4), play an important role in the development and progression of non-small cell lung cancer (NSCLC) by promoting cell growth and preventing apoptosis by regulating downstream effectors such as mitogen-activated protein kinase, protein kinase B, and signal transducer and activator of transcription 3 (1,2). Mutations in the kinase domain of ERBB2 gene have been reported to occur in lung adenocarcinomas (3-5) and lung squamous cell carcinomas (6) for some ethnicities, which could potentially result in the activation of the tyrosine kinase activity of ERBB2 protein and play a critical role in oncogenesis in a manner similar to EGFR mutations (7,8). However, to date, it is unclear that the status of ERBB2 mutations in Chinese patients with lung squamous cell carcinomas that are the majority of NSCLC.
To find out the overall status of ERBB2 mutations in Chinese patients with NSCLC, in this study we conducted a detailed search for the ERBB2 mutations in tumor tissues derived from a large cross-sectional cohort of Chinese patients with NSCLC who underwent tumor resection in several hospitals from southern China during a time period of 1.5 years.
Methods
Patients and tissue specimens
From June 2006 to November 2007, tumor tissues and paired normal lung tissues were obtained from 212 Chinese patients with NSCLC who underwent curative resection in the Tumor Hospital of Hunan Province and the Xiangya Hospital and the Xiangya 2nd Hospital of Central South University which are top three hospitals in Hunan Province, China. None of these patients had received chemotherapy or radiotherapy before surgery. Written informed consent was obtained from each patient before the surgery. This study was approved by the institutional review boards respectively from the Tumor Hospital of Hunan Province and the Xiangya Hospital and the Xiangya 2nd Hospital of Central South University. Pathological histology type was determined according to WHO criteria (9). All of the tumor and macroscopically normal lung tissue samples were obtained at the time of surgery, and then they were rapidly frozen in liquid nitrogen and stored at –80 °C. Only tumors with 80% or more tumor component were sent for DNA extraction and mutational analysis. The normal lung tissue specimens were obtained from either the opposite end of resected surgical samples or as distant as possible from the tumor. All of the macroscopically normal samples were confirmed as normal under hematoxylin and eosin staining.
Mutational analysis of ERBB2 gene
Genomic DNA was obtained from frozen lung tissues by overnight digestion with sodium dodecyl sulfate and proteinase K [Tiangen Biotech (Beijing) Co., Ltd, China] at 37 °C followed by standard phenole chloroform (1:1) extraction and ethanol precipitation. PCR-based direct sequencing and polymerase chain reaction-single strand conformational polymorphism (PCR-SSCP) were used to detect mutations of exons 19-20 of the TK domain of ERBB2 gene. The PCR was performed in a total volume of 25 µL containing 100 ng genomic DNA, 0.2 mmol/L of each primer, and 0.2 mmol/L dNTPs, 1 unit of Taq polymerase [Tiangen Biotech (Beijing) Co., Ltd, China], and 1 reaction buffer (10 mmol/L Tris-HCl, pH 8.3; 50 mmol/L KCl; and 1.5 mmol/L MgCl2). The PCR cycle conditions consisted of an initial denaturation step at 95 °C for 5 minutes followed by 30 cycles of 30 seconds at 95 °C and 60 seconds at 72 °C, and a final elongation at 72 °C for 7 minutes. The primers used for exon 19 were: forward, 5'-TGG AGG ACA AGT AAT GAT CTC CTG G-3' and reverse, 5'-AAG AGA GAC CAG AGC CCA GAC CTG-3', amplifying a 160-bp fragment; and for exon 20 were: forward, 5'-GCC ATG GCT GTG GTT TGT GAT GG-3' and reverse, 5'-ATC CTA GCC CCT TGT GGA CAT AGG-3', amplifying a 249-bp fragment. The PCR products were purified using a PCR Products Purification kit (Bio Basic Inc., CA), then sequencing was done using an ABI Prism 3100 Genetic Analyzer [Sangon Biotech (Shanghai) Co., Ltd, China]. Sequence chromatograms were analyzed by Mutation Surveyor 2.60, followed by manual review. At the same time, PCR-SSCP analysis was also performed for each sample. The purified PCR products were diluted 1:5 in loading buffer (95% formamide, 2 mM EDTA, pH 8.3), and then the diluted samples (12 µL) were denatured (5 min at 90 °C), immediately cooled on ice and loaded into a 12% non-denaturing polyacrylamide gel. Electrophoresis was carried out for 4 hr at 15 °C at 5 Watt. Upon completing migration the gels were subject to staining using ethidium bromide for 20 minutes.
Results
Patient characteristics
Between June 2006 and November 2007, tumor specimens suitable for genetic analysis were available from 212 patients with NSCLC. The cohort included patients with age at diagnosis ranging from 41 to 77 (median age, 63) in 172 males and from 33 to 72 (median age, 57) in 40 females. There were 153 Squamous cell carcinomas (72.2%), 49 adenocarcinomas (23.1%) and 10 adenosquamous carcinomas (4.7%) in the cohort including 148 smokers (69.8%) and 64 never-smokers (30.2%).
The status of ERBB2 mutation
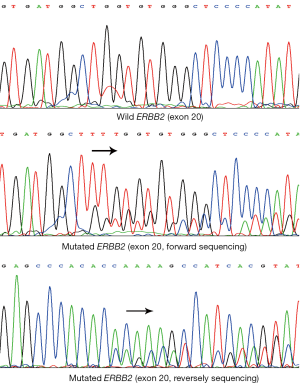
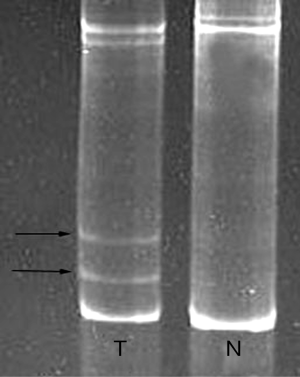
The ERBB2 mutation was found in 1 of 49 lung adenocarcinomas (2.0%), and none in 153 lung squamous cell carcinomas and 10 lung adenosquamous carcinomas. The single adenocarcinoma case with ERBB2 mutation was a never-smoker, female and 44 years of age. The category of ERBB2 mutation was a heterozygous in-frame insertion occurring within exon 20: 2327-2329insTTT (G776V, insC) (Figure 1). Likewise, PCR-SSCP analysis revealed no more than one ERBB2 mutation within exon 20 in the study cohort. The mutated ERBB2 presented two different electrophoresis bands distinguished from normal one (Figure 2).
Discussion
In the present study, we found only 1 ERBB2 mutation in 49 lung adenocarcinomas (2.0%) and no ERBB2 mutation in lung squamous cell carcinomas and lung adenosquamous carcinomas. The rate of ERBB2 mutation in this study is lower than those of previous researches (10,11), which didn’t involve lung squamous cell carcinomas and lung adenosquamous carcinomas. This difference may be due to the different distributions of sex and smoking status of study sample. In addition, the differences of environment and histological types should be considered too. Similar to our findings, Yokoyama et al. didn’t find ERBB2 mutations in Japanese lung squamous cell carcinomas (12).
As with EGFR mutations, ERBB2 mutations, which may be a therapeutic target in lung cancer patients (13), are more common in Asians, adenocarcinomas, females, and never-smokers (4,5,14). The patient with ERBB2 mutation in this study was never-smoker, female and adenocarcinomas. Thus, ERBB2 mutations may play a key role in the development of adenocarcinomas in nonsmokers. A 3-bp in-frame insertion mutation within exon 20 in our patients is not the major type of mutation found by other investigators (3-5,13), which may be resulted from a small sized study sample, but it is consistent with those of Shigematsu and colleagues (4). Further investigation will be needed to clarify the relationship between ERBB2 mutations and clinicopathologic features in Chinese patients with NSCLC.
In conclusion, our results suggest that ERBB2 mutations occur infrequently in Chinese patients with NSCLC, especially in lung squamous cell carcinomas. So it is possible of limited value for molecular target therapy based on the mutated ERBB2 in Chinese patients with NSCLC.
Acknowledgements
This study was supported by the Postdoctoral Foundation of China (No. 2012M511735, to SD Feng), and by the Natural Science Foundation of Hunan Province (No. 10JJ6046, to SD Feng). Meanwhile, we thank Professor Duanfang Liao and Director Bingyang Zhu, both of whom are from the Center for Life Science Research, University of South China, for their helps in this study.
Disclosure: The authors declare no conflict of interest.
References
- Seshacharyulu P, Ponnusamy MP, Haridas D, et al. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets 2012;16:15-31. [PubMed]
- Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol 2009;21:177-84. [PubMed]
- Stephens P, Hunter C, Bignell G, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature 2004;431:525-6. [PubMed]
- Shigematsu H, Takahashi T, Nomura M, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res 2005;65:1642-6. [PubMed]
- Sasaki H, Shimizu S, Endo K, et al. EGFR and erbB2 mutation status in Japanese lung cancer patients. Int J Cancer 2006;118:180-4. [PubMed]
- Lee JW, Soung YH, Kim SY, et al. ERBB2 kinase domain mutation in the lung squamous cell carcinoma. Cancer Lett 2006;237:89-94. [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- Travis WD, Brambilla E, Müller-Hermelink HK, et al. eds. World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. IARC Press, Lyon, 2004.
- Li C, Sun Y, Fang R, et al. Lung adenocarcinomas with HER2-activating mutations are associated with distinct clinical features and HER2/EGFR copy number gains. J Thorac Oncol 2012;7:85-9. [PubMed]
- Zhang Y, Sun Y, Pan Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res 2012;18:1947-53. [PubMed]
- Yokoyama T, Kondo M, Goto Y, et al. EGFR point mutation in non-small cell lung cancer is occasionally accompanied by a second mutation or amplification. Cancer Sci 2006;97:753-9. [PubMed]
- Cappuzzo F, Bemis L, Varella-Garcia M. HER2 mutation and response to trastuzumab therapy in non-small-cell lung cancer. N Engl J Med 2006;354:2619-21. [PubMed]
- Tomizawa K, Suda K, Onozato R, et al. Prognostic and predictive implications of HER2/ERBB2/neu gene mutations in lung cancers. Lung Cancer 2011;74:139-44. [PubMed]