Headway in resistance to endocrine therapy in breast cancer
Department of Breast Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing 100730, PR China
|
Review Article
Headway in resistance to endocrine therapy in breast cancer
Department of Breast Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing 100730, PR China
|
|
Abstract
Resistance to endocrine therapy is the major problem for ERα(+) breast cancer patients. Research in endocrine
resistance, mainly based on breast cancer cell lines and transplantation animal models, has indicated that phosphorylation
of estrogen receptors, high expression of SRC and high activation of ErbB/MAPK pathway are the
3 main mechanisms for occurrence of endocrine resistance. Restoration of ER expression and exploration of inhibitors
to various biological targets are the 2 promising ways to solve this problem. Further research is needed to
deeply explore relevant mechanisms and resolvents so as to guide clinical practice.
Key words
breast cancer; endocrine resistance; AEs/AIs; SRC; ErbB /MAPK
J Thorac Dis 2010;2:171-177. DOI: 10.3978/j.issn.2072-1439.2010.02.03.9
|
|
Introduction
Endocrine therapy of breast cancer dated back to the end
of 19th century when premenopausal breast cancer patients
began to receive curative bilateral ovariectomy. Tamoxifen
was used in pre- and post-menopausal breast cancer patients
since the 1980s, and it was better-tolerated than bilateral
ovariectomy. Several drugs (including TAM and AIs)
targeting ER are available now for ERα(+) breast cancer
patients in clinical settings, interfering internal environment
of breast cancer cells so as to inhibit tumor growth, reduce
recurrence rate and increase survival rate (1,2). Most ERα(+)
breast cancer patients could receive quite good effects
from endocrine therapy initially, however a certain tumors
would acquire resistance to endocrine therapy later and
recurrence and/or metastasis might occur (3-5). Resistance
to endocrine therapy is the major problem for ERα(+) breast
cancer patients, hence it’s of great importance to explore the
mechanism and countermeasures for dealing with resistance
to endocrine therapy. This review summarizes relevant
research in resistance to endocrine therapy and presents an
overview in this field.
|
|
Search method
Search strategy
Electronic searches were performed by databases of PubMed
from its inception to June 2010. To achieve the maximum
sensitivity of the search strategy and identify all studies about
breast cancer and endocrine therapy resistance, we used
appropriate free text and thesaurus terms including “breast
cancer”, “breast carcinoma”, “breast tumour”, “mammary
cancer”, “endocrine therapy”, “drug resistance”, “drug tolerance”
and all other relative information about breast. The MeSH
table was searched by “breast neoplasms” [MeSH Terms]
AND (“endocrine system”[MeSH Terms] AND “therapy”
[Subheading] OR “therapeutics” [MeSH Terms]) AND
resistance [All Fields]. The reference lists of all retrieved
articles were reviewed for further identification of potentially
relevant studies.
Study selection
All studies assessing breast cancer, endocrine therapy and
drug resistance published were included. No restrictions were
placed on abstracts and conference proceedings. We excluded
studies that were not directly relevant to drug resistance
to breast cancer endocrine therapy, such as resistance to
chemotherapy combined with endocrine therapy. Review
about drug resistance to breast cancer endocrine therapy
was excluded because we were about to explore the original
mechanisms and countermeasures to drug resistance to
breast cancer endocrine therapy, actually there is no reviews
concentrating on drug resistance to breast cancer endocrine
therapy.
|
|
Incidence of resistance to endocrine
therapy in breast cancer patients
Approximately 70% breast cancer patients are ERα(+) and
could get benefit from endocrine therapy through interfering
mitosis of tumor cells and inhibiting tumor growth (6-7).
TAM had been used in breast cancer endocrine therapy for
30 years. Many patients benefit from TAM while the problem
of endogenic and exogenetic resistance to TAM indeed exists
(8-9): among ERα(+) breast cancer patients, approximately
30%~40% have inherent resistance to TAM and can not
benefit from the use of TAM at all (6-7); approximately 62%
breast cancer patients, who take TAM orally after operation,
would need further surgery because of recurrence and/or
metastasis (10,11). The mechanism of fulvestrant (selective
estrogen receptor down-modulator) is different from that
of TAM, and breast cancer patients could benefit from
fulvestrant after they fail the treatment of TAM although
resistance to fulvestrant would eventually appear (12-14).
Compared with TAM, the AIs could inhibit tumor growth
more sustainably, but tumor cells would resist to AIs as well
finally (4). Hence many of the breast cancer patients treated
with endocrine therapies do not respond, and for those who
do, many acquire resistance over time (19). In a word, drug
resistance is the major problem of endocrine therapy, and it’s of
great importance to further explore it.
|
|
Research approaches of resistance to
endocrine therapy in breast cancer
Research based on breast cancer cell lines
Several breast cancer cell lines have been applied in research
of mechanism and countermeasures of drug resistance in
breast cancer endocrine therapy, for example, MCF-7 cell
line (6,15-17), MDA-MB-231cell line (6), LTLTCa cell line
(4) and T47D cell line (15). Under a certain process, those
cell lines could evolve various cell lines that have different
characteristics but all of them have resistance to breast cancer
endocrine therapy. For example, if cultured in medium rich in
OH-TAM for 6 months, MCF-7 breast cancer cell line would
evolve into CL6.8 cell strain, which has resistance to TAM (6);
if cultured in medium rich in OH-TAM and Fulvestrant, cell
strain resistant to OH-TAM and Fulvestrant would be evolved
(6). And MCF-7/HER2-18 is ER positive cell lines with HER2
over-expression, which was built by stable transfecting MCF-7
with over-expressed HER2; while MCF-7 wild type (MCFwt)
is positive ER cell lines without HER2 over-expression (19).
Through assays of cell growth and cell migration&invasion,
changes in different experimental group were evaluated
(15); through western blotting, relevant proteins’ level in cell
lines with or without resistance to endocrine drugs could be compared, hence the mechanism of endocrine resistance could
be further analysed (15). After inoculated with endocrineresponsive
cell lines (for example MCF-7Ca cells), mice could
be assigned into different groups when the tumors reached a
measurable size. The mice could be killed and tumor tissues
were collected with addition of endocrine drugs when: 1)
tumor volume began to shrink; 2) tumor volume stopped
shrinking and began to accrete; 3) tumor accreted to several
times of origin volume. Immuno-blotting and other methods
could be used to analyse relevant proteins’ level of these tumor
tissues and then the mechanism of endocrine resistance could
be further analysed (4).
Building animal models with endocrine
resistance
As to the problem of endocrine resistance, common research of
animal models in exploring mechanisms and countermeasures
is as follows: establishing animal models with endocrine
resistance through subcutaneous inoculation of tumor cells
with resistance to endocrine drugs, then in vivo observation
of tumor volume which would reveal the difference of various
drugs in inducing and reversing endocrine resistance to tumors
was performed. Usually when tumors reached a sufficient
size, for example, 150-200mm3, the animals were randomly
assigned to various treatment groups (19). Mice were
frequently chosen to build animal models (18-19), for example,
subcutaneous inoculation tumor cells in ovariectomized &
immunosuppressed mice so as to simulate the postmenopausal
breast cancer patients because the source of estrogen after
menopause is from nonovarian tissue and is not under
regulation by gonadotropins, which could be used to explore
mechanisms of endocrine resistance and countermeasures to
AIs (4). To explore mechanisms of endocrine resistance and
countermeasures to TAM, premenopausal animal models
could be built by subcutaneous heeling-in slow-release
estradiol pellets in ovariectomized mice which could simulate
the in vivo estrogen release (20). Ovariectomized athymic
nude mice in the presence of estrogen could also be used in
establishing xenografts (19). In vivo observation of resistancerelevant
proteins expression levels would promote thorough
analysis the mechanism of endocrine resistance.
|
|
Potential mechanisms of breast cancer
endocrine resistance
Various mechanisms are relevant to inducing endocrine
resistance (3). Different mechanisms have mutual correlation
with each other and cause endocrine resistance together,
while the molecular phenotype can change over time (19). Fig
1 conveys the basic signaling pathways and relevant targets
together with their inhibitors associated with endocrine resistance. The main biological targets of endocrine resistance
are summarized in the following chart:
Phosphorylation of estrogen receptors (ERs)
Phosphorylation is one of the post-translational modification
(PTMs) in cells including phosphorylation, glycosylation and
acetylization, and ER is the main target of phosphorylation
(21). Phosphorylation of ERs plays a pivotal role in acquiring
endocrine resistance to TAM (22). In TAM-resistant MCF-7
Her-2/neu breast cancer cell lines and TAM-resistant
breast cancer tumor tissues, it has been demonstrated by
western blotting that specific ligand-dependent endogenous
phosphorylation of ER occurs at S118 and S167 (22-25).
Phosphorylated ER would lose ligand-dependance. While
in v itro research revealed that different mechanisms
of phosphor ylation would lead to different biological
characteristics of ER: ER phosphorylation induced by steroid
receptor coactivator (SRC) and protein kinase A (PKA) would
increase ER’s affinity to estrogen (E2); ER phosphorylation
induced by mitogen-activated protein kinase (MAPK) would
reduce ER’s affinity to trans-hydroxytamoxifen (TOT) (22).
Phosphorylated-ER’s affinity to estrogen response elements
(ERE) would be significantly reduced with stimulation of
exogenous TOT no matter by which mechanisms ERs were
phosphorylated (22). With presence of E2, ER phosphorylation
induced by AKT, MAPK and PKA would all increase the mutual
interaction between DNA combining receptors and SRC3
receptors (22). Compared with TAM, although it takes a much
longer time for AIs to induce breast cancer endocrine resistance,
phosphorylation of ERs still plays an important role in acquiring
endocrine resistance to AIs (3,21). It has been demonstrated
that expression level of phosphorylated-ERα in tumor tissues
resistant to letrozole is much higher than that in control group,
which is sensitive to letrozole; while expression level of ERα(not
phosphorylated) is much lower than that of control group (4).
Meanwhile, expression level of phosphorylated-ERα will be
even higher in tumor tissues resistant to letrozole if tumors
continue growing under a certain concentration of letrozole, and
expression level of ERα (not phosphorylated) will be even lower,
while expression level of MAPK will be higher and expression
level of PR will be constant (4). There are many phosphorylation
sites of ERα, and different sites were phosphorylated by different
mechanisms (21).
Steroid receptor coactivator (SRC)
It has been stated above that SRC plays an important role in
the process of ER phosphorylation. SRC acts as a non-receptor
tyrosine kinase, and its overexpression has a close correlation
with various malignant tumor genesis including breast cancer
(26-27). In vitro research has revealed that SRC has correlation
with development of breast cancer endocrine resistance (17). Overexpression of SRC will weaken tumor cells’ sensitivity
to TAM in MCF-7 breast cancer cells which are with positive
ERα and sensitive to TAM; while using inhibitor of SRC could
reduce its expression and restore tumor cells’ sensitivity to
TAM in breast cancer endocrine resistant cells developed from
TAM-sensitive MCF-7 breast cancer cells. The expression level
of SRC in cytoplasma of breast cancer tumor cells is much
higher than that in cytoplasma of breast cells besides tumor
in human (p<0.01); while in nucleolus the expression level
of SRC is just the other way. This implies that the correlation
between the reactivity of breast cancer cells to endocrine drugs
and SRC expression levels in cytoplasma or nucleolus might be
poles apart. AZD0530 could inhibit SRC kinase activity dosedependently,
as shown by a decrease in phosphorylaiton of
SRC at Y419 (15). In addition, AZD0530 together with TAM
significantly suppress expression of the Ki-67 antigen, and
has correlation with expression of both cyclin-D1 (necessary
for the progression of cells from G1 to S phase) and C-Myc (a
positive regulator of cellular proliferation) (15).
ErbB family
The ErbB family has 4 members, including ErbB1, ErbB2,
ErbB3 and ErbB4, and all of them are tyrosine kinase receptors.
Research conducted by Ghayad et al (6) has demonstrated
that ErbB1, ErbB2 and ErbB3 are activated and ErbB4 is highly
expressed in endocrine resistant breast cancer cells, while
ErbB heterodimers and various ligands relevant to ErbB are
also highly expressed. AKT and MAPK are the main biological
targets in the downstream of ErbB family associated signal
transduction pathway, and activated AKT and MAPK have a
close correlation with breast cancer endocrine resistance (16).
The activated AKT has correlation not only with endocrine
resistance but also with poor prognosis (28). In breast
cancer cell lines with resistance to TAM and Fulvestrant,
the MAPK pathway is highly activated (29-30), which will
make ERαfurther phosphorylated and make cells even more
resistant to endocrine drugs through various ways (31,32). It
has been demonstrated by western blotting that the biological
target MAPK and PI3K/AKT are activated in breast cancer
cells resistant to endocrine drugs, while highly expressed
MAPK has close correlation with phosphorylation of serine
at site 118 in region AF1 of ERα, and highly expressed PI3K/
AKT has close correlation with phosphorylation of serine at
site 167 in region AF1 of ERα (6). In clinical settings, patients
usually benefit less from TAM if the tumors are with positive
ERαand MAPK highly phosphorylated (33).
Fig 1 The basic signaling pathways and relevant targets together with their inhibitors associated with endocrine resistance Estrogen receptor alpha (ER) and the growth factors (esp. Her-1 and Her-2) are the two main tumor markers used in the clinic to help predicting therapeutic response in breast cancer. The classical estrogen signaling pathway is responsible for growth, and TAM and AI and F are usually used to treat ER positive patients. TAM is selective estrogen receptor modulator inhibiting the combination of E2 and ER. AI could inhibit the peripheral convertion of other agents to E2. F could down-regulate the expression level of ER. Growth factor signaling via EGFR and Her-2 and stress-related pathways associated with p38 and ERK1,2 mitogen activated protein kinases have relationship with de novo and acquired resistance to endocrine therapy (19). Various treatments could be used in exploring the mechanisms of endocrine resistance, for example, E2 with the EGFR tyrosine kinase inhibitor gefitinib (E2 +G), estrogen deprivation (ED), estrogen deprivation plus the antiestrogen tamoxifen (ED+TAM),ED plus TAM and gefitinib (ED+TAM+G) or ED plus fulvestrant (ED+F), et al. SRC plays an important role in endocrine resistance, because it is the cross target for the two main signaling pathways associated with endocrine resistance. AZD0530 and TAM together showed improved growth inhibitory effects compared with either agent alone, and they could prevent the emergence of tamoxifen resistance; at the highest concentration of AZD0530(1μM), it will show corresponding inhibition of MAPK activity in the MCF-7 and T47D cell lines (15). In addition various targets and relevant inhibitors are shown in this figure.
Abbr: TAM, tamoxifen; ER, estrogen receptor; E2, estrogen; EGF, epithelial growth factor; Her-1(EGFR), epithelial growth factor receptor; G, gefitinib; F, fulvestrant (ER down-regulator); AI, aromatase inhibitor; MAPK, mitogen activated protein kinases; H, herceptin; ERK, extracellular signal-regulated kinase; MMP, matrix metalloproteinase; ADAM, a disintegrin and metalloproteinase; AREG, amphiregulin; PI3K, phosphatidylinositol-3-kinase. |
|
Outlook of resolution of endocrine
resistance
Restoration of ER expression
Breast cancer patients with negative ERα will not benefit
from endocrine therapy at all, which is primary resistant to
endocrine resistance. Breast cancer patients with positive
ERα will benefit from endocrine drugs at first, but will acquire
resistance to it later, which is secondary resistant to endocrine
therapy. Primary resistance to endocrine therapy is mainly
due to methylation of promoter in ERα encoding gene and
remodeling of chromatin, which is quite different from the
mechanisms of secondary resistance, however there are some
relationship between primary and secondary resistance to
endocrine therapy.
The down-regulation of ERα expression induced by highly
activated MAPK can be reversed: inhibition of MAPK
activity would make ERα expression up-regulated; and ERα
expression would be down-regulated again if the activity of
MAPK is restored (7). Research conducted by Brinkman
et al demonstrated that the mechanisms of ERα expression
deficiency has correlation not only with methylation of
promoter in ERα encoding gene but also with high activation
of MAPK induced by EGFR and ErbB2 overexpression.
Hence MAPK could be selected as the potential target for reexpression
of ERα and restoration of sensitivity to endocrine
therapy (34). A clinical trial including 10 negative ERα and
positive ErbB2 breast cancer patients demonstrated that
3 patients became to be positive ERα and then acquired
continuous sensitivity to letrozone after intravenous injection
of Herceptin for a period of time (35). In transplantation
animals based on positive ERα and positive ErbB2 MCF-7
breast cancer cell lines, high expression of MAPK is associated
with deficiency of ERα expression, while ERα would be reexpressed
and sensitivity to endocrine therapy would be
restored if inhibitor of MAPK was applied (18).
Combination of various drugs
Based on mechanisms of endocrine resistance, inhibitors
of different biological targets could be used in treatment of
breast cancer alone or in combination, which might suppress
the occurrence of endocrine resistance and restore sensitivity
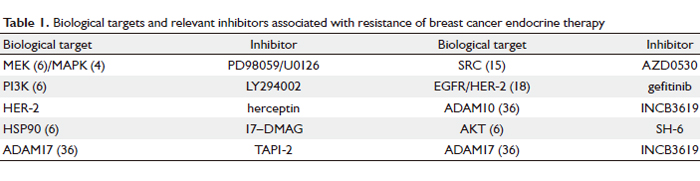
to endocrine drugs. Table 1 below shows various biological
targets and relevant inhibitors associated with resistance
of breast cancer endocrine therapy. Various inhibitors to biological targets are mainly used in basic research while
scarcely used in clinical settings, and much more research
is needed to develop drugs which could be used in clinical
practice.
EGFR-MAPK is the main target associated with breast
cancer endocrine therapy, and activation of EGFR is regulated
by various cytokines, including EGF, TGF–α, ADAM17/
AREG, HB–EGF, BTC, epiregulin, epigen and so on (20).
After EGFR is activated by the above mentioned cytokines,
downstream of the pathway will be activated by forming EGFR
homodimers or forming heterodimers with ErbB2, ErbB3 and
ErbB4. Combination of endocrine drugs and EGFR/ ErbB2
inhibitors would be a novel regimen which might solve the
problem of endocrine resistance and elevate therapeutic effects
(18).
For LTLTCa cells, which are separated from tumor tissues
with resistance to letrozole, the expression level of ERα could
be restored to the original status and sensitivity to AIs and
AEs could also be restored if it is cultured in medium rich in
Herceptin, which is inhibitor of ErbB2 signal pathway (37).
This indicates that there are intimate crosstalk between the
signal pathways of ER and ErbB2. However, compared with
the patients who take letrozole alone as adjuvant therapy,
those who take letrozole and Herceptin in combination
show no better prognosis, which indicates that combination
of Herceptin and endocrine drugs would be a better choice
than each one alone in patients with recurrence or metastasis,
therefore in adjuvant settings taking Herceptin and endocrine
drugs in combination is not recommended (4). At present,
phase Ⅲ clinical trial is carried out to explore the difference
between neratinib group and neratinib plus AIs group in
patients with recurrence or metastasis. Besides, AZD0530,
which is inhibitor of SRC, combined with TAM could
effectively prevent the occurrence of endocrine resistance
based on research in breast cancer cell lines, indicating that
SRC probably is the potential target of preventing occurrence
of endocrine resistance (15).
In summary, endocrine resistance is one of the problems for
ERa positive breast cancer patients. Research on mechanisms
of endocrine resistance mainly focused on one signal pathway.
As mechanisms of endocrine resistance involve multiple signal pathways and multiple targets, and there are complicated
crosstalks among those pathways, therefore further research
work is needed to further explore relevant mechanisms so as to
guide clinical practice.
 |
|
References
Cite this article as: Xu YL, Sun Q. Headway in resistance to endocrine therapy in breast cancer. J Thorac Dis 2010;2(3):171-177. doi: 10.3978/j.issn.2072-1439.2010.02.03.9
|