Indwelling pleural catheter infection and colonisation: a clinical practice review
Introduction
Pleural effusions are the accumulation of fluid in the space between the lung and chest wall and are commonly caused by cancer (malignant pleural effusions, MPEs), though other benign causes include heart, liver or renal failure. MPEs signify advanced cancer and are associated with significant morbidity and mortality (1,2). Indwelling pleural catheters (IPCs) are an effective treatment for breathlessness caused by MPEs, and are also used in treatment-refractory, benign pleural effusions (3,4). The most significant and common complication of IPC usage is infection (5,6). In this review, we discuss the incidence, microbiology, pathology and management of IPC infection and colonisation.
Definitions
IPC infections refer to microbial growth on an IPC or associated pleural fluid, leading to infective signs and symptoms. In contrast, IPC colonisation describes microbial growth in pleural fluid that does not cause a clinically evident infection.
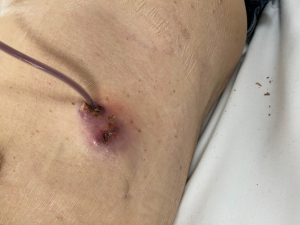
IPC infections can be divided into superficial or deep (Table 1). However, many terms have been used to describe categories of IPC infection (5,7-10). The American Association for Bronchology and Interventional Pulmonology (AABIP) noted the lack of universally applicable definitions used to describe IPC infections and categorized infections according to the site of infection, as described in Table 1 (7,8). Subsequent guidelines simplified this further, proposing just two broader categories of infection: superficial infections (including cellulitis, exit-site and catheter-tract infection; Figure 1) and deep, pleural space infections (9). For the purposes of this review, we will describe IPC infections as superficial or deep, as defined in Table 1.
Table 1
| Classification | Definition |
|---|---|
| Superficial infection | |
| Cellulitis | Redness, warmth, oedema, and mild pain of the skin and immediate subcutaneous tissue |
| Exit-site infections | Purulent drainage at the catheter-epidermal interface, which can be associated with induration and erythema of the catheter tract, localized to the exit site |
| Tunnel tract infections | Erythema, oedema, induration and tenderness along the catheter tract, >2 cm proximal from the exit site |
| Deep infection | |
| Pleural space infection | Exists when any of the following are present: (I) obvious pus drained from the catheter; (II) presence of clinical symptoms consistent with infection with positive pleural fluid Gram stain or culture; (III) presence of clinical symptoms consistent with infection along with pleural fluid biochemical analysis supportive of infection, such as elevated lactate dehydrogenase, low glucose or low pH |
| IPC colonisation | Positive pleural fluid or catheter segment bacterial cultures, in the absence of any evidence of infection |
IPC, indwelling pleural catheter.
IPC colonisation is defined as the presence of positive bacterial culture from pleural fluid drained via an IPC, in the absence of any signs or symptoms of infection. This has been likened to colonisation of a urinary catheter (11). For non-draining IPCs, we have defined colonisation as bacterial growth from catheter segments that were not associated with a clinical infection (12). It has not always been recognised that IPCs may become colonised, and distinguishing between infection and colonisation can be challenging. IPC colonisation may be misinterpreted as deep infection and unnecessarily treated with antibiotics. In clinical studies, deep infection is often defined as positive pleural fluid cultures and treatment with antibiotics (13). This misunderstanding may lead to a falsely elevated estimation of the incidence of deep infection.
Incidence
Data on the incidence of IPC infection comes from published randomised trials and observational cohort studies. However, determining an accurate incidence is limited by inconsistent definitions of IPC infection. A recent meta-analysis found the incidence of IPC infections to be 5.7% [95% confidence interval (CI): 4.0–7.7%] (5). Superficial infections were divided into wound infection (incidence 0.4%, 95% CI: 0.1–1.0%) and cellulitis (incidence 0.9%, 95% CI: 0.3–1.7%). Deep infections were divided into pleural infection (incidence 0.6%, 95% CI: 0.1–1.3%), and empyema (incidence 1.3%, 95% CI: 0.6–2.2). Another review estimated an overall rate of infection of 5.8% (95% CI: 5.1–6.7%), with a rate of superficial infection of 1–4.9% and of deep infection of 0.6–12.6% (6). This data demonstrates that deep infections are more common than superficial infections.
The incidence of IPC colonisation is not known. A recent observational study found 8 of 41 (19.5%) patients had IPC colonisation, where colonisation was defined by positive pleural fluid cultures (14). The ongoing ‘Bacteria Responsible for IPC Infection and Colonisation’ (BRICC) study has collected IPCs following their removal (12). Of 60 IPCs that we have collected to date (out of a target of 100), 10 were associated with an infection. From the remaining 50, bacteria were grown from an intrathoracic portion of 17 IPCs (34%, data unpublished). This preliminary data demonstrates that in a significant proportion of cases IPCs become colonised.
Microbiology
The microbiology of IPC colonisation has not been studied extensively. The organisms identified as colonisers in the aforementioned cohort study were Coagulase-negative Staphylococci (CoNS), various anaerobes (including Bacillus species), Streptococcus mitis, Brevibacterium species, Corynebacterium and Candida species (14). From our own, unpublished data we have sequenced bacterial isolates from six colonised IPCs. All were colonised by CoNS, with one also colonised by Corynebacterium jeikeium.
A wide range of organisms can cause IPC infection, of which the most common is Staphylococcus aureus (Table 2). This data comes from two studies: a retrospective cohort study of 50 IPC infections and a further analysis of data from a meta-analysis (15,16). The key difference between these studies is that 12/76 (15.8%) of infections in the meta-analysis were caused solely by CoNS, compared to 2/50 (4%) in the retrospective cohort study. As discussed earlier, IPC colonisation may be misdiagnosed as infection. This means that some of the bacteria identified in Table 2 as being infective organisms may actually have been colonising the IPC, particularly CoNS. This misdiagnosis may account for the difference in frequency of CoNS between these two studies.
Table 2
| Organism | Frequency seen | Percentage of infections (n=124) |
|---|---|---|
| Staphylococcus aureus | 47 | 37.9% |
| Coagulase-negative Staphylococci | 18 | 14.5% |
| Culture negative | 12 | 9.7% |
| Klebsiella spp. | 8 | 6.5% |
| Escherichia coli | 8 | 6.5% |
| Pseudomonas aeruginosa | 8 | 6.5% |
| Viridans streptococci | 5 | 4.0% |
| Serratia marcescens | 4 | 3.2% |
| Corynebacterium spp. | 4 | 3.2% |
| Enterococcus spp. | 4 | 3.2% |
| Proteus mirabilis | 2 | 1.6% |
| Bacteroides spp. | 2 | 1.6% |
| Acinetobacter baumannii | 2 | 1.6% |
| Stenotrophomonas maltophilia | 2 | 1.6% |
| Diphtheroids | 2 | 1.6% |
| Enterobacter cloacae | 2 | 1.6% |
| Haemophilus influenzae | 1 | 0.8% |
| Listeria monocytogenes | 1 | 0.8% |
| Streptococcus pyogenes | 1 | 0.8% |
| Streptococcus agalactiae | 1 | 0.8% |
| Staphylococcus lugdunesis | 1 | 0.8% |
| Cutibacterium acnes | 1 | 0.8% |
| Escherichia hermannii | 1 | 0.8% |
| Candida | 1 | 0.8% |
| Mycobacterium fortuitum | 1 | 0.8% |
Whilst helpful to make a diagnosis of an IPC infection, positive microbiological identification of an organism is not essential. In 9.7% of patients treated for an IPC infection, no organism identified was identified using conventional culture-based methods. These infections are described as ‘culture-negative’. 16s rRNA sequencing is not universally available nor routinely used to identify organisms, though it may help provide further insights into the causes of infection.
In summary, S. aureus and CoNS are the most commonly cultured organisms in IPC associated deep infection and IPC colonisation respectively. Knowledge of which organisms most often cause IPC infections ensures appropriate empiric antibiotic treatment and may be able to help distinguish between infection and colonisation.
Mechanism
We hypothesize that biofilm formation upon an IPC’s surface is central to the deep infection and colonisation of IPCs. Biofilms are complex bacterial communities, able to adhere to foreign bodies and human tissues (17,18). They play a major role in human disease, and biofilm infections are particularly difficult to treat. Bacteria within a biofilm are encased within extracellular polymeric substances, comprised of proteins, polysaccharides and extracellular DNA. This impairs antimicrobial penetrance through the bacterial population. Additionally, the properties of bacteria within a biofilm can differ significantly from its planktonic form, with differential gene expression, a greater capacity for plasmid conjugation (gene transfer between bacteria) and the ability to slow their metabolic rate, limiting the efficacy of antimicrobials (17,18). Once a biofilm has formed upon a foreign body, it is difficult to clear without its removal (19).
There is some evidence that biofilms play a significant role in deep IPC infections. Infections develop weeks-to-months following insertion (10,11,20,21), hence are rarely caused by poor sterile technique during catheter insertion, and are more likely to reflect subsequent contamination of an IPC during handling and drainage. The BRICC study has demonstrated that bacteria are present on cultured segments of IPCs (12). Further study of the panel of bacteria acquired from colonised and infected IPCs will help identify virulence factors, such as genes associated with antimicrobial resistance or biofilm production. The same study has conducted laboratory studies which demonstrate that relevant bacterial species readily form biofilms on IPC segments grown in donated human pleural fluid (unpublished observations). Biofilm growth on removed IPCs has also been detected. There needs to be further work to support this hypothesis, particularly work demonstrating the presence of biofilms on colonised and infected IPCs. An understanding of how commonly used treatments affect biofilms on IPCs is also important.
An alternative hypothesis is that infections arise from the migration of commensal skin bacteria along the outside of the catheter tract. However, the presence of a polyester cuff induces local fibrosis and closure of the catheter tract following IPC insertion. This is purported to help prevent infection, though it is unclear how quickly the IPC tract closes. Furthermore, deep infection is rarely associated with corresponding superficial infection. The AMPLE-4 study presupposes this is the main cause of infection and attempts to reduce infection rates by using topical mupirocin (14).
Another possibility is that bacterial translocation following a pneumonia may lead to IPC infection. However, pneumonia preceding an IPC infection has rarely been described. Additionally, the microbiology of IPC infections is quite distinct to that of pneumonia.
In summary, there is some evidence that suggests deep IPC infections and colonisation are associated with bacterial biofilm formation on the IPC surface.
Risk factors
Risk factors for IPC infection are hepatic hydrothorax (compared to other indications) and length of time that the IPC has been in situ. In a subgroup analysis, Wang et al. found a pooled probability of IPC infection of 12.6% (95% CI: 8.1–17.8%) in patients with hepatic hydrothorax compared to 0.7% (95% CI: 0.0–4.5%) in patients with heart failure (5). Another meta-analysis including only patients treated with IPC for hepatic hydrothorax found a deep infection rate of 12.4% (22). Multiple reasons for the increased risk of IPC infection when used to manage hepatic hydrothorax have been postulated. These include relative immune and bone marrow suppression common in liver disease, and the possibility of ‘spontaneous bacterial empyema’. This describes the translocation of enteric bacteria into the pleural space (23,24).
An observational cohort study of risk factors for IPC infection in patients with MPE undergoing antineoplastic therapy found a median time from IPC insertion to infection of 41 days and that longer IPC duration was associated with a hazard ratio of 1.03 (95% CI: 1.00–1.06, P=0.028) for infection (21). Length of time that the IPC has been in situ is also associated with IPC colonisation (12).
Clinicians are often concerned that chemotherapy increases the risk of IPC infection due to immunosuppression. Reassuringly, a recent meta-analysis demonstrated that systemic cancer therapy does not increase the risk of IPC infection, even in immunocompromised patients with moderate or severe neutropenia (relative risk 0.98, 95% CI: 0.93–1.03) (25).
Diagnosis
The prompt recognition and diagnosis of IPC infections is important to manage patients effectively. Clinicians must carefully interpret biochemistry and clinical features, along with microbiological results, which can be unreliable due to IPC colonisation.
Diagnosis of superficial infection is a clinical diagnosis based on the appearance of the skin around the IPC site (Table 1, Figure 1). Carers who drain IPCs should look out for these signs and alert medical staff promptly if concerned. A swab of any discharge from around the IPC should be taken to identify the bacterial species responsible and to identify which antibiotics it is sensitive to, but antibiotics should not be withheld pending culture results. Clinicians should consider thoracic ultrasound to detect areas of septation or loculation and sending a sample of pleural fluid, given that superficial and deep infections may co-exist.
Patients with deep IPC infections may present with change in the appearance of the pleural fluid drained e.g., cloudy fluid or frank pus. Patients or their carers should be aware that they should contact their pleural team urgently if they notice a change in the appearance of fluid drained and should be reviewed promptly in clinic.
Deep infection should also be suspected in patients with an IPC and signs and symptoms of infection. These patients are frequently immunosuppressed, so other sources of infection must be considered. Samples of fluid should be sent for microscopy, culture and sensitivity (MC&S). However, positive cultures may be due to colonisation rather than infection. Identification of bacteria which typically cause infection (e.g., S. aureus) rather than colonisation (e.g., CoNS) may help distinguish between these causes of positive pleural fluid cultures. Blood cultures should also be sent in order to aid organism identification if the patient is suspected to have a bacteraemia.
The specificity of pleural fluid cultures for infection may be aided by obtaining a direct pleural fluid sample via thoracocentesis rather than via the IPC (8). It may be helpful to also check pleural fluid lactate dehydrogenase (LDH), pH and glucose levels. LDH is usually raised in both MPE and pleural infection, whereas pH and glucose levels are reduced. Therefore, these tests are non-specific, but a significant change in these values compared to previous results suggests infection (26).
Blood should be sent to assess inflammatory markers (e.g., white cell count, C-reactive protein levels). A procalcitonin can also be requested if available. Pleural fluid levels of soluble urokinase plasminogen activator receptor (SuPAR) have been demonstrated to be higher in patients requiring more aggressive pleural infection treatment, in those without an IPC (27). Its role in deep IPC infections has not yet been evaluated.
In conclusion, prompt recognition of signs and symptoms of infection in combination with appropriate sampling for MC&S are the key elements of diagnosis of IPC infection.
Management
Antibiotics are the mainstay of treatment for both deep and superficial IPC infection, but deep infection also requires drainage of infected pleural fluid and may need IPC removal (8,9,28,29). In contrast, colonisation does not require treatment.
Patients with IPC infections can generally be managed as outpatients. Clinically unstable patients (evidence of shock or sepsis), those not improving despite appropriate care as an outpatient and those requiring additional procedures should be admitted (8,9). Outpatients should be reviewed regularly in clinic to ensure that infection is resolving.
Superficial infections are treated with a short course of oral antibiotics until skin changes resolve, usually 7 to 10 days. Antibiotic choice should be based on local guidelines for treatment of cellulitis (typically an oral penicillin) and then refined based on results of skin swab MC&S. IPCs can remain in place during initial management, but should be removed if infection fails to resolve with adequate antibiotic treatment (i.e., persistent clinical evidence of cellulitis) (9). IPC removal should be avoided where possible, as the underlying pleural effusion still requires ongoing drainage, hence another IPC may be needed. In a series of 448 IPCs, only 2/14 (14%) superficial infections required IPC removal (20).
Deep infections require longer courses of antibiotics, around 4 to 6 weeks. Antibiotic course duration depends on treatment response, serum inflammatory marker response and whether adequate drainage is achieved (8,9). Initial antibiotic choice should be based on local guidelines for treatment of pleural infection and then can be further refined based on culture results, bearing in mind that infections may be polymicrobial with both aerobic and anaerobic bacteria. Patients are typically managed with penicillin antibiotics that have anaerobic cover, such as co-amoxiclav.
Deep infections require drainage of infected pleural fluid via the IPC, usually by admission and use of a portable suction device or underwater seal drain (8,9,28). If the patient is not septic or they are coming to the end of their life and it is a priority to stay at home, then daily home IPC drainage can be used to manage infection (9). IPC removal and replacement with another chest drain is not usually needed during the acute phase of infection but may be necessary in patients who do not respond to initial treatment, have poor drainage via the IPC or if they have a concurrent tract infection (8,28). For the reasons outlined above, IPC removal should also be avoided if possible.
When drainage via the IPC is inadequate, fibrinolytics and DNAse (intrapleural enzyme therapy, IET) given via the IPC should be considered. IET is used to improve drainage in pleural infections that are not associated with IPCs, but there is some evidence for its use in patients with IPC-associated deep infections (4,30). A multi-centre retrospective study of IET in 39 IPC-associated deep infections demonstrated good drainage volumes without major morbidity or mortality (26). IET helps drain infected fluid and may also help to breakdown biofilms on the IPC, aiding bacterial clearance. We use a dose of alteplase 10 mg (or urokinase 100,000 IU if alteplase is unavailable) and Dornase alfa 5 mg administered intrapleurally via the IPC for up to three days. This can be given twice daily in inpatients or once daily in outpatients. Imaging (with chest X-ray, ultrasound or CT) is useful to detect residual fluid which has not drained.
Surgery is not usually an option for patients with IPC infection due to frailty from the underlying disease necessitating IPC insertion. Immunosuppressive treatment, such as chemotherapy, should be stopped while there is active infection but can be resumed once infection settles (9).
In summary, antibiotics are used to treat both deep and superficial infections, with the addition of IPC drainage for deep infection. IPC removal should be avoided during treatment of acute infection when pleural fluid is draining and IET can be considered for select patients.
Outcomes
Whereas superficial infections usually resolve with oral antibiotic treatment, deep infections may result in pleurodesis, resolution of the infection with ongoing drainage, chronic infection or death. Pleurodesis describes the adherence of the parietal and visceral pleura, preventing further fluid buildup and permitting IPC removal.
Fysh et al. found that 62% of patients underwent spontaneous pleurodesis following a deep infection, though this was higher for patients with a S. aureus infection (78.6% vs. 45.0%, P=0.04) (15). Similarly, Frost at al noted that following a deep infection 11 MPEs treated with an IPC, 10 had undergone pleurodesis (20). This is thought to be a consequence of the local inflammatory reaction that occurs with infection.
Deep IPC infections have a mortality of 2.2–6% (15,31), compared to 20% in pleural infection not associated with IPCs (32). Estimating the mortality of IPC infections is challenging, as this cohort have concurrent, life-limiting disease. Therefore, death may be attributed to the underlying illness rather than infection, leading to an underestimation in the mortality of IPC infections.
Prevention
The morbidity caused by IPC infection means prevention is important. We recommend use of a dedicated procedure room with full body draping during IPC insertion. Prophylactic antibiotics given at the time of insertion do not reduce infection rates (33). Aseptic non-touch technique during drainage prevents inoculation of bacteria into the IPC (14,34-36). Topical mupirocin is used to prevent infection in peritoneal dialysis catheters and the AMPLE-4 trial (a randomised controlled trial of mupirocin versus placebo) is currently recruiting to study whether this can also reduce IPC infection (14). Patients are also advised to ensure that the IPC insertion site is kept clean and dry, with activities such as swimming avoided for a month following insertion.
The patient perspective
A survey of 84 patients with IPCs demonstrated that 23% of patients worry about getting an infection in their IPC (37). Concerns about IPC infection means some patients choose not to have one sited. Furthermore, some patients are reluctant to self-manage their IPC because they are concerned that there is a greater infection risk if they or a family member drains it, as compared with a health care professional.
Future directions
Better understanding of IPC infection and colonisation will lead to a decrease in incidence and improved treatment of infection. The BRICC Study will provide further details regarding the incidence and microbiology of IPC colonisation (12). Our current work is investigating the relationship between biofilm formation and IPC infection. Future IPCs may have a biofilm resistant surface to prevent biofilm formation and hence reduce the incidence of infection.
A better understanding of the relationship between colonisation and infection should help clinicians distinguish between these and prevent unnecessary antibiotic treatment. Further work is required to provide clinicians with more confidence in interpreting positive microbiology, particularly when distinguishing colonisation from infection. Some clinicians opt to perform a thoracocentesis to obtain a pleural fluid sample that has not been collected from the IPC to rule-out colonisation. This approach is yet to be validated and future studies should specifically examine this relationship (8). Further studies need to be done in infection prevention, of which AMPLE-4 is the first large randomized controlled trial (RCT) to study this problem. Future work should also study the role of IET in treatment of deep infection.
IPC infections occur infrequently meaning that large sample sizes are required to adequately power a study aimed at reducing their incidence. A composite endpoint of IPC infection or colonisation may make smaller studies feasible, but it is unclear what impact this would have on the clinical utility of a study’s findings.
Conclusions
IPC infections affect around 5% of patients and are most commonly caused by S. aureus. Deep infection can usually be managed with antibiotics and drainage of infected material. It commonly results in pleurodesis and mortality is low. In contrast, IPC colonisation is usually caused by CoNS and does not require treatment. We hypothesize that colonisation and infection are associated with bacterial biofilm formation on IPCs and that a better understanding of this relationship could lead to better treatment and infection prevention strategies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Avinash Aujayeb) for the series “Malignant and Benign Pleural Effusions” published in Journal of Thoracic Disease. The article has undergone external peer review.
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1761/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1761/coif). The series “Malignant and Benign Pleural Effusions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 2014;69:1098-104. [Crossref] [PubMed]
- Penz E, Watt KN, Hergott CA, et al. Management of malignant pleural effusion: challenges and solutions. Cancer Manag Res 2017;9:229-41. [Crossref] [PubMed]
- Frost N, Ruwwe-Glösenkamp C, Raspe M, et al. Indwelling pleural catheters for non-malignant pleural effusions: report on a single centre's 10 years of experience. BMJ Open Respir Res 2020;7:e000501. [Crossref] [PubMed]
- Roberts ME, Rahman NM, Maskell NA, et al. British Thoracic Society Guideline for pleural disease. Thorax 2023;78:s1-s42. [Crossref] [PubMed]
- Wang S, Zhang R, Wan C, et al. Incidence of complications from indwelling pleural catheter for pleural effusion: A meta-analysis. Clin Transl Sci 2023;16:104-17. [Crossref] [PubMed]
- Sundaralingam A, Bedawi EO, Harriss EK, et al. The Frequency, Risk Factors, and Management of Complications From Pleural Procedures. Chest 2022;161:1407-25. [Crossref] [PubMed]
- Miller CRJ, Chrissian AA, Lee YCG, et al. Key Highlights From the American Association for Bronchology and Interventional Pulmonology Evidence-Informed Guidelines and Expert Panel Report for the Management of Indwelling Pleural Catheters. Chest 2021;159:920-3. [Crossref] [PubMed]
- Miller RJ, Chrissian AA, Lee YCG, et al. AABIP Evidence-informed Guidelines and Expert Panel Report for the Management of Indwelling Pleural Catheters. J Bronchology Interv Pulmonol 2020;27:229-45. [Crossref] [PubMed]
- Gilbert CR, Wahidi MM, Light RW, et al. Management of Indwelling Tunneled Pleural Catheters: A Modified Delphi Consensus Statement. Chest 2020;158:2221-8. [Crossref] [PubMed]
- Chalhoub M, Saqib A, Castellano M. Indwelling pleural catheters: complications and management strategies. J Thorac Dis 2018;10:4659-66. [Crossref] [PubMed]
- Lui MM, Thomas R, Lee YC. Complications of indwelling pleural catheter use and their management. BMJ Open Respir Res 2016;3:e000123. [Crossref] [PubMed]
- Sethi DK, Rhodes J, Webber MA, et al. S5 Bacterial isolates from infected and non-infected indwelling pleural catheters. Thorax 2022;77:A7.
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs. chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Lau EPM, Faber S, Charlesworth C, et al. Topical antibiotics prophylaxis for infections of indwelling pleural/peritoneal catheters (TAP-IPC): A pilot study. Respirology 2024;29:176-82. [Crossref] [PubMed]
- Fysh ETH, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest 2013;144:1597-602. [Crossref] [PubMed]
- Sethi D, Webber M, Mishra EK. The microbiology of indwelling pleural catheter infections. Clin Transl Sci 2023;16:1287-8. [Crossref] [PubMed]
- Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis 2002;8:881-90. [Crossref] [PubMed]
- Vestby LK, Grønseth T, Simm R, et al. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics (Basel) 2020;9:59. [Crossref] [PubMed]
- Roy R, Tiwari M, Donelli G, et al. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018;9:522-54. [Crossref] [PubMed]
- F Frost N. Indwelling pleural catheters for malignancy-associated pleural effusion: report on a single centre's ten years of experience. BMC Pulm Med 2019;19:232. [Crossref] [PubMed]
- Wilshire CL, Chang SC, Gilbert CR, et al. Association between Tunneled Pleural Catheter Use and Infection in Patients Immunosuppressed from Antineoplastic Therapy. A Multicenter Study. Ann Am Thorac Soc 2021;18:606-12. [Crossref] [PubMed]
- Avula A, Acharya S, Anwar S, et al. Indwelling Pleural Catheter (IPC) for the Management of Hepatic Hydrothorax: The Known and the Unknown. J Bronchology Interv Pulmonol 2022;29:179-85. [Crossref] [PubMed]
- Shojaee S, Rahman N, Haas K, et al. Indwelling Tunneled Pleural Catheters for Refractory Hepatic Hydrothorax in Patients With Cirrhosis: A Multicenter Study. Chest 2019;155:546-53. [Crossref] [PubMed]
- Kniese C, Diab K, Ghabril M, et al. Indwelling Pleural Catheters in Hepatic Hydrothorax: A Single-Center Series of Outcomes and Complications. Chest 2019;155:307-14. [Crossref] [PubMed]
- Porcel JM, Cordovilla R, Tazi-Mezalek R, et al. Efficacy and Safety of Indwelling Catheter for Malignant Pleural Effusions Related to Timing of Cancer Therapy: A Systematic Review. Arch Bronconeumol 2023;59:566-74. [Crossref] [PubMed]
- Fitzgerald DB, Muruganandan S, Tsim S, et al. Intrapleural Fibrinolytics and Deoxyribonuclease for Treatment of Indwelling Pleural Catheter-Related Pleural Infection: A Multi-Center Observational Study. Respiration 2021;100:452-60. [Crossref] [PubMed]
- Arnold DT, Hamilton FW, Elvers KT, et al. Pleural Fluid suPAR Levels Predict the Need for Invasive Management in Parapneumonic Effusions. Am J Respir Crit Care Med 2020;201:1545-53. [Crossref] [PubMed]
- Bedawi EO, Ricciardi S, Hassan M, et al. ERS/ESTS statement on the management of pleural infection in adults. Eur Respir J 2023;61:2201062. [Crossref] [PubMed]
- Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of Malignant Pleural Effusions. An Official ATS/STS/STR Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:839-49. [Crossref] [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- Gilbert CR, Lee HJ, Skalski JH, et al. The Use of Indwelling Tunneled Pleural Catheters for Recurrent Pleural Effusions in Patients With Hematologic Malignancies: A Multicenter Study. Chest 2015;148:752-8. [Crossref] [PubMed]
- Davies HE, Davies RJ, Davies CW, et al. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii41-53. [Crossref] [PubMed]
- Nuttall E, Balata H, Al-Aloul M, et al. P182 Prophylactic doxycycline following Indwelling Pleural Catheter insertion for malignant pleural effusions. Thorax 2015;70:A168.
- Akram MJ, Khalid U, Bakar MA, et al. Indications and clinical outcomes of indwelling pleural catheter placement in patients with malignant pleural effusion in a cancer setting hospital. Clin Respir J 2020;14:1040-9. [Crossref] [PubMed]
- Gilbert CR, Lee HJ, Akulian JA, et al. A Quality Improvement Intervention to Reduce Indwelling Tunneled Pleural Catheter Infection Rates. Ann Am Thorac Soc 2015;12:847-53. [Crossref] [PubMed]
- Aujayeb A. P02.18 Indwelling Pleural Catheter Placement in a Large UK District General Hospital. J Thorac Oncol 2021;16:S254.
- Mitchell MA, Deschner E, Dhaliwal I, et al. Patient perspectives on the use of indwelling pleural catheters in malignant pleural effusions. Thorax 2023;78:1111-7. [Crossref] [PubMed]