
Comparison of safety and effectiveness of different sheaths in ablation of focal atrial tachycardia: a retrospective study
Highlight box
Key findings
• Utilizing the visible steerable sheath (Vizigo sheath) for the ablation of focal atrial tachycardia (FAT) can significantly improve the rate of successful ablation.
What is known and what is new?
• Catheter ablation has emerged as a first-line treatment option in patients with recurrent or incessant FAT but recurrence rates remain high.
• Using the Vizigo sheath improves long-term arrhythmia-free survival while also decreasing procedural fluoroscopy times.
What is the implication, and what should change now?
• The Vizigo sheath offers distinct advantages in the ablation of FAT.
Introduction
Focal atrial tachycardia (FAT) is a kind of cardiac arrhythmia that accounts for up to 10% of supraventricular tachycardias (1). A female preponderance has been described, with a twofold higher risk in women than men (2). FAT may also trigger other atrial arrhythmias, such as atrial flutter and atrial fibrillation (AF) (3,4). Catheter ablation (CA) has emerged as a first-line treatment option in patients with recurrent or incessant FAT (2). Nevertheless, recurrences remain common with one study reporting 58% freedom from arrhythmia without antiarrhythmic drugs (AADs) at 12 months of follow-up (5). Refining ablation procedures and techniques, therefore, carries the potential to improve outcomes. Steerable sheaths have been demonstrated to improve the clinical effectiveness of ablation in AF (6,7).
A novel bidirectional steerable sheath was recently developed that allows for three-dimensional visualization without fluoroscopy (Carto Vizigo; Biosense Webster, Irvine, CA, USA; Figure 1). While using the CARTO navigation system, the sheath is accurately displayed by the combination of a magnetic sensor on the catheter and an electrode on the sheath; allowing the direction of the sheath and its relationship with the catheter to be precisely visualized. In addition to the potential to improve ablation efficacy due to superior lesion formation (8,9), such a tool also carries the potential to decrease radiofrequency time and radiation exposure (8-11). Direct visualization on Electroanatomic Mapping systems, combined with a seamless transition from the tip to the dilator, enables smooth entry into the left atrium (LA) during transseptal access, eliminating the necessity for additional fluoroscopy. Considering the above advantages of the Vizigo sheath, there are few studies on its use in FAT ablation.
We, therefore, sought to compare the efficacy and safety of a visualized bidirectional Vizigo sheath to a non-visualized steerable sheath (Agilis NxT sheath; Abbott Laboratories, Abbott Park, IL, USA) and other conventional sheaths in the ablation of FAT. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-52/rc).
Methods
Study population
In this retrospective cohort study, 164 consecutive patients undergoing a first CA procedure for FAT at West China Hospital, Sichuan University from March 2019 to February 2022 were enrolled as participants. All patients were offered ablation with the Vizigo sheath. However, a total of 122 patients refused Vizigo sheaths due to financial reasons. Because we studied FAT patients, non-FAT patients were excluded. Patients whose baseline data could not be collected or who were lost to follow-up were excluded. None of the patients enrolled had concomitant ablation for AF. The study was carried out in compliance with the Declaration of Helsinki (as revised in 2013), and evaluated and approved by the Ethics Committee of West China Hospital, Sichuan University [No. 2022(257)]. The requirement for informed consent was waived because the retrospective nature of this study and patient information was de-identified and anonymized before the analysis.
Baseline characteristics
Patients were categorized into three groups according to whether a Vizigo sheath, non-visualized steerable sheath, or other conventional sheath was used. Other conventional sheath is mainly composed of fixed sheath (Swartz sheath; St. Jude Inc., St. Paul, MN, USA). Baseline characteristics recorded at the time of ablation included age, sex, electrocardiographic (ECG) parameters, cardiovascular history, body mass index (BMI), left atrial diameter (LAD), left ventricular diameter (LVD), and left ventricular ejection fraction (LVEF). Transthoracic echocardiography and standard blood tests were systematically performed prior to the procedure. Antiarrhythmic medications other than amiodarone were discontinued for at least 5 half-lives prior to the procedure.
Electrophysiology study and CA
All procedures were performed in the fasting awake state, with minimal conscious sedation. The vascular sheath was placed via femoral venous access. A quadripolar catheter was positioned in the right ventricle and a 10-pole catheter was placed in the coronary sinus as a reference and for pacing. When a left atrial origin was suspected, the LA was accessed by means of a single transseptal puncture, and an activated clotting time of greater than 300 s was maintained throughout the intervention. The ablation catheter (THERMOCOOL SMARTTOUCH SF, Biosense Webster Inc., Irvine, CA, USA) was inserted through the Vizigo sheath, non-visualized steerable sheath, or standard sheath and guided using a 3D electroanatomic mapping system [CARTO 3, Johnson & Johnson (J&J) MedTech, New Brunswick, NJ, USA]. Upon confirmation of the diagnosis of FAT, 3D reconstruction of the chamber of interest [right atrium (RA) or LA] was performed.
Irrigated radiofrequency ablation of the FAT was performed with a power limit of 40 W, maximum temperature of 50 ℃, and flow limit of 20 mL/min. Try ablation was attempted in the RA with a power of 10–30 W. The power level depends on the patient’s tolerance. The catheter was repositioned and ablation was reattempted if the lesion was ineffective or the FAT recurred. Acute success was defined as tachycardia termination with no FAT recurrence within 30 min of ablation, along with the inability to induce FAT despite an intravenous infusion of isoproterenol with programmed electrical stimulation. The overall procedural duration, ablation time, fluoroscopy time, contact force (CF; average, minimum, and maximum), and acute ablation success were recorded. Procedural duration was defined as the time from the start of mapping to the end of ablation. All procedures were performed by 4 experienced cardiac electrophysiologists who had a CA volume of >500 procedures per year at West China Hospital. The primary safety outcomes refer to complications related to surgery, including puncture, cardiac tamponade, bradycardia, atrioesophageal fistula, and stroke, which we have added in the original manuscript.
Patient follow-up
After being monitored for 24 hours following the procedure, patients were discharged barring a procedure-related complication. Patients were systematically followed-up at our arrhythmia clinic every 3–6 months for the first year after hospital discharge, and then yearly thereafter. All patients received 100 mg aspirin tablet daily for 3 months after the procedure. The presence/absence of atrial tachycardia (AT) during follow-up was evaluated by symptoms, ECG recordings, and 24-hour Holter monitoring. Long-term success was defined as complete remission of the patient’s symptoms without the use of AADs and with no AT recorded on ECGs or 24-hour Holter monitors.
Statistical analysis
Statistical analysis was performed using the software SPSS 26.0 (IBM Corp., Armonk, NY, USA). Normally-distributed continuous variables were expressed as mean ± standard deviation (SD). When the distribution was not normal, median and interquartile ranges (IQRs) were provided. Frequencies and percentages were used to summarize categorical data. Three-group comparisons of continuous variables were performed using one-way ANOVA or Kruskal-Wallis tests depending on whether the data were normally distributed or skewed. Two-group comparisons were performed using independent t-tests (normal distribution) or Mann-Whitney U tests (nonnormal distribution). Categorical variables were compared using chi-square or Fisher’s exact tests where appropriate. Two-tailed P values <0.05 were considered to indicate statistical significance.
Results
Participant characteristics
A total of 164 patients, mean age 50±15 years, 97 (59.1%) women, were included in this study. In all, 42 (25.6%) patients were allocated to the Vizigo sheath, 36 (22.0%) to a non-visualized steerable sheath, and 86 (52.4%) to other conventional sheaths. Baseline characteristics are summarized in Table 1. In 49 (29.9%) individuals, AT originated from the LA and in the remainder, it originated from the RA. The site of origin of AT was as follows: tricuspid valve 26, pulmonary veins 28, bundle of His region 12, RA septum 10, LA septum 11, LA roof 10, superior vena cava 8, coronary sinus 25, crista terminalis 21, and mitral valve 13. Age, sex, BMI, presence of hypertension, history of heart failure, and prevalence of diabetes mellitus were not significantly different among the three groups. By echocardiography, there were no significant differences in LVD, LAD, and LVEF.
Table 1
| Characteristics | Vizigo sheath (n=42) | Non-visualized steerable sheath (n=36) | OCS sheath (n=86) | P value |
|---|---|---|---|---|
| Age (years) | 53±16 | 50±19 | 49±16 | 0.37 |
| Male | 18 (42.9) | 14 (38.9) | 35 (40.7) | 0.94 |
| BMI (kg/m2) | 23.5±3.2 | 22.5±3.3 | 23.3±3.2 | 0.36 |
| Hypertension | 9 (21.4) | 6 (16.7) | 14 (16.3) | 0.76 |
| Diabetes II | 5 (11.9) | 3 (8.3) | 7 (8.1) | 0.57 |
| Heart failure | 6 (14.3) | 4 (11.1) | 13 (15.1) | 0.91 |
| LAD (mm) | 35 [34–41] | 35 [32–37] | 34 [33–38] | 0.17 |
| LVD (mm) | 47 [47–49] | 47 [45–48] | 47 [45–50] | 0.75 |
| LVEF | 64 [63–68] | 63 [61–71] | 64 [62–68] | 0.96 |
| RAD (mm) | 36 [32–39] | 33 [30–36] | 34 [30–37] | 0.36 |
Data were presented as mean ± standard deviation, n (%), or median [interquartile range]. OCS, other conventional sheath; BMI, body mass index; LAD, left atrium diameter; LVD, left ventricular diameter; LVEF, left ventricular ejection fraction; RAD, right atrial diameter.
Acute and long-term success
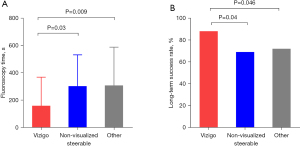
Acute ablation success was achieved in 154 (94.0%) patients, with no difference between groups. Over an average follow-up of 14±2 months after ablation, 124 (75.6%) patients remained arrhythmia-free. The long-term success rate was 88.1% in patients with the Vizigo sheath, which was significantly higher than in those with a non-visualized steerable sheath (69.4%, P=0.04) and other standard sheaths (72.1%, P=0.046).
Procedural data
Procedural durations were not significantly different among the three groups: 32 (IQR, 21–47), 37 (IQR, 24–53), and 34 (IQR, 26–55) min for patients with the Vizigo sheath, non-visualized steerable sheath, and other conventional sheaths, respectively. As shown in Table 2, there were likewise no significant differences in the number of ablation lesions and ablation time among these groups. However, the fluoroscopy time was shorter with the Vizigo sheath [145 (IQR, 40–380) s] compared to non-visualized steerable sheaths [250 (IQR, 141–414); P=0.03] and other conventional sheaths [302 (IQR, 151–439); P=0.009; Figure 2].
Table 2
| Characteristics | Vizigo sheath (n=42) |
Non-visualized steerable sheath (n=36) | OCS sheath (n=86) |
P value | |
|---|---|---|---|---|---|
| Vizigo vs. non-visualized steerable sheath | Vizigo vs. OCS sheath |
||||
| Left atrial tachycardia | 18 (42.9) | 10 (27.8) | 23 (26.7) | 0.17 | 0.07 |
| Procedure time (min) | 32 [21–47] | 37 [24–53] | 34 [26–55] | 0.65 | 0.74 |
| Fluoroscopy time (s) | 145 [40–380] | 250 [141–414] | 302 [151–439] | 0.03 | 0.009 |
| Local activation time (ms) | 27 [34–42] | 34 [28–45] | 29 [26–42] | 0.68 | 0.67 |
| Ablation time (s) | 141 [208–469] | 173 [90–428] | 220 [116–319] | 0.58 | 0.49 |
| Ablation lesions | 6 [9–25] | 8 [5–17] | 9 [6–12] | 0.79 | 0.16 |
| The minimum CF >10 g | 36 (85.7) | 23 (63.9) | NR | 0.03 | NR |
Data were presented as n (%) or median [interquartile range]. OCS, other conventional sheath; CF, contact force; NR, not reported.
Compared with the non-visualized steerable sheaths, the Vizigo sheath was associated with a higher average CF [12.0 (IQR, 9.0–12.7) vs. 8.2 (IQR, 6.8–10.2) g; P=0.003; Figure 3A]. Similar results were obtained with regard to minimum [10.0 (IQR, 6.0–12.5) vs. 5.0 (IQR, 4.0–7.8); P<0.001; Figure 3B] and maximum CF [15.0 (IQR, 11.5–19.5) vs. 11.0 (IQR, 10.0–13.0); P=0.007; Figure 3C]. The proportion of patients with a minimum CF >10 g was higher in those with Vizigo compared to non-steerable sheaths (85.7% vs. 63.9%; P=0.025).
Complications
Overall, 3 (1.8%) patients experienced complications, all of whom had non-steerable conventional sheaths. Complications consisted of 2 hematomas and 1 third degree atrioventricular block. There was no death, atrioesophageal fistula, pericardial effusion, or stroke.
Discussion
To our knowledge, this is the first study to systematically assess the impact of the Vizigo sheath on CA outcomes in patients with FAT. Main findings include the superiority of the Vizigo sheath over non-visualized steerable or other conventional sheaths with regard to the following outcomes: (I) arrhythmia-free survival; (II) average, minimum, and maximum CF, and (III) shorter fluoroscopy time.
Prior multicenter studies have shown that ablation of FAT is effective in improving symptoms, with 81% of patients reporting no or fewer symptoms at the 12-month mark following CA. However, recurrences are common with freedom from arrhythmia without AAD noted in 58% (56% for left AT vs. 62% for right AT), with lower rates in patients with biatrial arrhythmias (5). In our more contemporary study, a success rate of 71.3% at 12 months was achieved without the Vizigo sheath (3,12,13), which was further improved to 88.1% with the Vizigo sheath.
We speculate that the increased success rate can be explained by the following reasons. In contrast to Busch et al.’s study in which 3D mapping systems were seldom used, this tool was systematically used in our cohort (5). Moreover, emerging technologies such as multipoint high-density mapping and panoramic mapping were employed when needed in difficult cases. Above and beyond these technological advances, the Vizigo sheath provided an incremental benefit. When assessing the efficacy of CA, CF is a key consideration. The multi-center EFFICAS I study demonstrated that the minimum CF achieved for ablation significantly predicted ablation gaps and ineffective lesions (14). In current practice, a CF >10 g can help reduce recovery of pulmonary vein electrical conduction (15). The proportion of patients that achieved a minimum CF >10 g in the Vizigo sheath group in our study was significantly higher than the proportion of patients with this endpoint in the non-steerable sheath group. Minimum, average, and maximum CFs were higher with the Vizigo sheath. These results are consistent with our earlier study in patients with AF that demonstrated that the Vizigo sheath dramatically increased the average CF and the proportion of patients with a CF within a reasonable range when compared to the fixed sheath (8). Concordantly, Rajendra et al. reported that the Vizigo sheath was linked to a 10% improvement in catheter stability (P=0.0005) (11). All of these findings provide a biologically plausible mechanism to support the increased rate of arrhythmia-free survival. Whereas a small reduction in CF could be compensated by an increase in ablation time, no statistically significant differences in ablation times were observed among the patients enrolled in the 3 groups in our study.
At present, the visible steerable sheath has been applied on a large scale in AF ablation, and several studies have reported an increase in catheter stability, reduction in radiation exposure, and shortened post-procedural hospital length of stay in a Chinese real-world hospital setting, without compromising safety (6,7,16,17). Additionally, precise manipulation is possible because of real-time deflection and visual monitoring of the Vizigo sheath’s motion. Catheter and sheath positioning might also facilitate and accelerate mapping and ablation, for example, by ensuring a close alignment between the sheath and catheter tip, resulting in improved control and catheter tip-to-tissue CF during ablation. This capability may enhance catheter stability in problematic locations.
Since employing non-visualized steerable or other conventional sheaths typically demands periodic fluoroscopy imaging to determine the position of the sheath and catheter, the need for fluoroscopy can be reduced by a sheath that can be viewed by a 3D mapping system. According to several studies, 3D mapping and intracardiac echocardiography (ICE) can successfully treat AF without fluoroscopy (18,19). According to Tahin et al., ICE-guided zero-fluoroscopic AF ablation could be successfully implemented in routine practice in the electrophysiology laboratories (19). During FAT ablation, the mean fluoroscopy time was reduced by 40% in patients with the Vizigo sheath, which is similar to the performance of Vizigo sheath in AF ablation (8,9,11). Visualizing a sheath in a 3D mapping system provides superior 3D appreciation for the anatomical location of the sheath and catheter in comparison to standard monoplane or orthogonal biplane fluoroscopic views. This may be particularly helpful in the ablation of FAT in which precise localization of the focal source is critical to its successful elimination. A reduction in fluoroscopy exposure is compatible with the ALARA (“as low as reasonably achievable”) principle in order to minimize risks to the patient, operator, and assisting staff to the well-known harmful stochastic effects of radiation (20-22).
Limitations
The study is single-center and retrospective. It merits confirmation by larger prospective multicenter studies, preferably with random allocation of the CA sheath. Ascertainment of arrhythmia-free survival was performed according to standard clinical, ECG, and ambulatory arrhythmia monitoring. Asymptomatic AT events occurring between two visits may have escaped detection. It is, therefore, possible that a more aggressive follow-up regimen would have yielded a higher recurrence rate. Further studies are required to assess longer-term outcomes. Our study included only single-source FAT such that it should not be generalized to patients with multiple arrhythmias.
Conclusions
The visible steerable sheath (Vizigo) has distinct advantages for FAT CA. The application of Vizigo sheath can improve the long-term success rate of FAT and reduce the radiation exposure of patients and medical staff in our single-center limited sample study.
Acknowledgments
We thank Dr. Christoph Sinning (University Heart & Vascular Center Hamburg, Hamburg, Germany) for the critical comments and valuable advice on this study.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-52/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-52/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-52/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-52/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study conformed to the principles of the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of West China Hospital, Sichuan University [No. 2022(257)]. The requirement for informed consent was waived because the retrospective nature of this study and patient information was de-identified and anonymized before the analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Porter MJ, Morton JB, Denman R, et al. Influence of age and gender on the mechanism of supraventricular tachycardia. Heart Rhythm 2004;1:393-6. [Crossref] [PubMed]
- Brugada J, Katritsis DG, Arbelo E, et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardiaThe Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J 2020;41:655-720. [Crossref] [PubMed]
- Chen SA, Chiang CE, Yang CJ, et al. Sustained atrial tachycardia in adult patients. Electrophysiological characteristics, pharmacological response, possible mechanisms, and effects of radiofrequency ablation. Circulation 1994;90:1262-78. [Crossref] [PubMed]
- Kalman JM, Olgin JE, Karch MR, et al. "Cristal tachycardias": origin of right atrial tachycardias from the crista terminalis identified by intracardiac echocardiography. J Am Coll Cardiol 1998;31:451-9. [Crossref] [PubMed]
- Busch S, Forkmann M, Kuck KH, et al. Acute and long-term outcome of focal atrial tachycardia ablation in the real world: results of the german ablation registry. Clin Res Cardiol 2018;107:430-6. [Crossref] [PubMed]
- Zhao Y, Zhang C, Peng L, et al. Clinical effectiveness and efficiency of a new steerable sheath technology for radiofrequency ablation in Chinese patients with atrial fibrillation: a retrospective comparative cohort study. J Thorac Dis 2023;15:3953-64. [Crossref] [PubMed]
- Luo Q, Xie Y, Bao Y, et al. Utilization of steerable sheath improves the efficiency of atrial fibrillation ablation guided by robotic magnetic navigation compared with fixed-curve sheath. Clin Cardiol 2022;45:482-7. [Crossref] [PubMed]
- Guo R, Jia R, Cen Z, et al. Effects of the visualized steerable sheath applied to catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol 2022;64:511-8. [Crossref] [PubMed]
- Khalaph M, Sommer P, Lucas P, et al. First clinical experience using a visualized sheath for atrial fibrillation ablation. Pacing Clin Electrophysiol 2022;45:922-9. [Crossref] [PubMed]
- Wakamatsu Y, Nagashima K, Kurokawa S, et al. Impact of the combined use of intracardiac ultrasound and a steerable sheath visualized by a 3D mapping system on pulmonary vein isolation. Pacing Clin Electrophysiol 2021;44:693-702. [Crossref] [PubMed]
- Rajendra A, Hunter TD, Morales GX, et al. Steerable sheath visualizable under 3D electroanatomical mapping facilitates paroxysmal atrial fibrillation ablation with minimal fluoroscopy. J Interv Card Electrophysiol 2023;66:381-8. [Crossref] [PubMed]
- Manolis AS, Lazaridis K. Focal atrial tachycardia ablation: Highly successful with conventional mapping. J Interv Card Electrophysiol 2019;55:35-46. [Crossref] [PubMed]
- Hu YF, Higa S, Huang JL, et al. Electrophysiologic characteristics and catheter ablation of focal atrial tachycardia with more than one focus. Heart Rhythm 2009;6:198-203. [Crossref] [PubMed]
- Neuzil P, Reddy VY, Kautzner J, et al. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol 2013;6:327-33. [Crossref] [PubMed]
- Park CI, Lehrmann H, Keyl C, et al. Mechanisms of pulmonary vein reconnection after radiofrequency ablation of atrial fibrillation: the deterministic role of contact force and interlesion distance. J Cardiovasc Electrophysiol 2014;25:701-8. [Crossref] [PubMed]
- Fitzpatrick N, Mittal A, Galvin J, et al. The impact of steerable sheath visualization during catheter ablation for atrial fibrillation. Europace 2023;25:1345-51. [Crossref] [PubMed]
- Ghosn M, Elsakka AS, Ridouani F, et al. Augmented fluoroscopy guided transbronchial pulmonary microwave ablation using a steerable sheath. Transl Lung Cancer Res 2022;11:150-64. [Crossref] [PubMed]
- Hang F, Cheng L, Liang Z, et al. Study on the Curative Effect and Safety of Radiofrequency Catheter Ablation of Paroxysmal Atrial Fibrillation via Zero-Fluoroscopy Transseptal Puncture under the Dual Guidance of Electroanatomical Mapping and Intracardiac Echocardiography. Cardiol Res Pract 2021;2021:5561574. [Crossref] [PubMed]
- Tahin T, Riba A, Nemeth B, et al. Implementation of a zero fluoroscopic workflow using a simplified intracardiac echocardiography guided method for catheter ablation of atrial fibrillation, including repeat procedures. BMC Cardiovasc Disord 2021;21:407. [Crossref] [PubMed]
- Casella M, Dello Russo A, Russo E, et al. X-Ray Exposure in Cardiac Electrophysiology: A Retrospective Analysis in 8150 Patients Over 7 Years of Activity in a Modern, Large-Volume Laboratory. J Am Heart Assoc 2018;7:e008233. [Crossref] [PubMed]
- Faroux L, Blanpain T, Nazeyrollas P, et al. Effect of Modern Dose-Reduction Technology on the Exposure of Interventional Cardiologists to Radiation in the Catheterization Laboratory. JACC Cardiovasc Interv 2018;11:222-3. [Crossref] [PubMed]
- Wartofsky L. Increasing world incidence of thyroid cancer: increased detection or higher radiation exposure? Hormones (Athens) 2010;9:103-8. [Crossref] [PubMed]




