Robot-assisted minimally invasive esophagectomy versus minimally invasive esophagectomy for thoracic lymph node dissection in patients with squamous cell carcinoma: a retrospective comparative cohort study
Highlight box
Key findings
• By comparing minimally invasive esophagectomy (MIE) cohort with robot-assisted minimally invasive esophagectomy (RAMIE) cohort, our research also shows that the transthoracic approach in RAMIE cohort yielded a greater total number of thoracic lymph nodes dissected and a greater extent of lymph node dissection that underwent thoracic lymph node dissection. Moreover, RAMIE is not associated with additional surgical complications.
What is known and what is new?
• Much clinical research has shown that differences in the number of lymph nodes removed between RAMIE cohort and MIE cohort.
• Previous studies have reported a difference in the total number of lymph node dissections in RAMIE cohort over MIE cohort. Our study further compares the differences in lymph node clearance in different regions of the thoracic cavity between the two cohorts.
What is the implication, and what should change now?
• RAMIE may result in better clearance of chest lymph nodes than MIE without more postoperative complications. Prospective studies are needed to validate this.
Introduction
Esophageal cancer (EC) ranks seventh in incidence among cancers and is the sixth leading cause of cancer-related death, resulting in approximately 509,000 deaths annually (1). Despite the increase in adenocarcinoma incidence, esophageal squamous cell carcinoma (ESCC) remains the predominant histologic type of EC globally and is most prevalent in Iran, Central Asia, and China. In Asia, ESCC accounts for more than 90% of EC. It is the most frequent pathological subtype of EC in China, constituting over 90% of all cases (2,3). In nonmetastatic cases, 5-year survival rates from 20% to 35% have been reported (4).
Minimally invasive esophagectomy (MIE) has become the favored surgical approach across the world, as its minimally invasive nature provides superior postoperative outcomes (5). However, primary tumors are usually located in the middle of the esophagus, close to the trachea, aorta, and other vital organs (6) and may cause a wide range of lymph node metastases. Moreover, MIE has certain technical limitations in resecting lymph nodes. A European study showed that a small thoracic cage width is significantly correlated with longer operation time during the thoracic phase of MIE, which suggests increased surgical difficulty (7). The advantages of robot-assisted minimally invasive esophagectomy (RAMIE), such as the high-definition three-dimensional (3D) vision and the presence of the EndoWrist (Intuitive Surgical Inc., Sunnyvale, CA, USA), facilitate movement in challenging anatomical regions (8). Therefore, examining the outcomes of lymph node dissection in the thoracic segment of EC across different approaches may be an important area of research. Results from high-volume centers are encouraging: total MIE (including MIE and RAMIE), compared to the open or hybrid approach, is associated with a lower overall, pulmonary, cardiac, and wound complication rate as well as a shorter hospital stay (9). There are reports indicating that RAMIE has an advantage in the number of lymph nodes dissected compared to MIE (6,9,10). However, few studies have examined more detailed groupings in thoracic lymph node dissection, especially for comparing RAMIE with MIE in terms of postoperative outcomes in patients with ESCC.
The aim of this study was thus to investigate the possible advantages of RAMIE over MIE for thoracic lymph node dissection in terms of postoperative outcomes. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-201/rc).
Methods
Patients
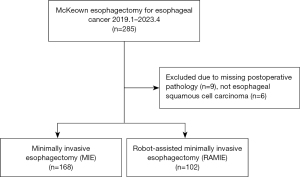
Clinical data for this retrospective comparative cohort study were obtained from the Esophageal Mediastinum Ward of the Thoracic Surgery Department of Harbin Medical University Cancer Hospital. We identified 285 patients with ESCC who had undergone surgical resection between January 2019 and April 2023. The included patients were operated upon by the same experienced operator to minimize bias. This operator has performed over 500 robotic-assisted surgeries and has specialized in EC for more than 10 years. Patients with ESCC who underwent RAMIE/MIE-McKeown esophagectomy for thoracic lymph node dissection were included. Patients were excluded if they had (I) missing postoperative pathology data or (II) a postoperative pathology of nonsquamous cell carcinoma (Figure 1). Finally, 270 patients who met the criteria were enrolled in our study. The patients were then divided into two groups according to the thoracic approach: MIE (n=168) or RAMIE cohort (n=102). The RAMIE cohort had less than 3 years of follow-up, so the study did not include long-term survival outcomes. We retrospectively collected data on patient characteristics and postoperative complications from their medical records. The following patient data were collected: gender, age, body mass index (BMI), smoking, alcohol, tumor location, diabetes, hypertension, tumor pathology type, neoadjuvant therapy program, surgical methods, surgical procedure, extent of lymph node dissection, pathological-stage, postoperative pathological thoracic lymph node outcome, and selected perioperative outcomes.
Pathological-stage was determined according to the eighth edition of the American Joint Committee on Cancer staging system (11,12). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Harbin Medical University Cancer Hospital (No. 2020-50-IIT), and all patients provided their written informed consent.
Surgical procedure
All surgeries were performed under general anesthesia with selective intubation to block the right lung. Most operations in the hospital included two-field lymph node dissection with an anastomosis in the neck. All patients received totally minimally invasive McKeown esophagectomy by the same experienced surgeon, and 78.1% of patients underwent lymph node dissection of the bilateral recurrent laryngeal nerves regardless of the procedure type. The chest cavity was inflated using carbon dioxide insufflation at a pressure of 6–8 mmHg. In all cases, the anastomosis was sewn at the neck using circular anastomosis. All patients in this study underwent a one-stage operation with gastric conduit reconstruction.
The lymph nodes are evaluated according to their anatomical location and numbered, which is done by the operator and his first and second assistants. After removal of the lymph nodes, the lymph nodes were placed in corresponding specimen bags according to their numbering. These numbered lymph nodes were included with the esophago-gastrectomy specimen for postoperative pathology if rapid pathology was not requested separately.
For the resection of the thoracic lymph nodes, it was necessary to open the mediastinal pleura, clear the lymph nodes adjacent to the right recurrent laryngeal nerve [106recR], dissect the arch of the chiasm, and free the esophagus up to the thoracic inlet and down to the esophageal diaphragmatic hiatus. We then continued to clear the upper thoracic periesophageal lymph nodes [105], the left recurrent laryngeal nerve lymph nodes [106recL], the paratracheal lymph nodes [106pre], the tracheobronchial lymph nodes [106tb], the left tracheobronchial lymph nodes [106tbL], the right tracheobronchial lymph nodes (106tbR), the sublongitudinal lymph nodes [107], the midthoracic periesophageal lymph nodes [108], the main bronchial lymph nodes [109], the subthoracic periesophageal lymph nodes [110], the supradiaphragmatic lymph nodes [111], the posterior mediastinal lymph nodes [112], the thoracic anterior aortic lymph nodes [112aoA], and the inferior pulmonary ligament lymph nodes [112pul]. An intraoperative rapid pathology of the right cervical paraesophageal lymph nodes [101R] were required, and when the intraoperative rapid pathology findings of 101R were positive, the right cervical lymph nodes needed to be removed.
Postoperative complications
Postoperative complications were categorized using the Clavien-Dindo (CD) classifications as follows: grade I was defined as any deviation from the normal postoperative course without the need for pharmacologic treatment or surgical, endoscopic, or radiologic intervention; grade II was defined as pharmacologic treatment with drugs; grade III was defined as surgical, endoscopic, or radiologic intervention; grade IV was defined as life-threatening complications that required intensive care unit (ICU) management; and grade V was defined as mortality (13).
Statistical analysis
Statistical analysis was conducted using SPSS 26.0 (IBM Corp., Armonk, NY, USA) and R version 3.6.3 (The R Foundation for Statistical Computing, Vienna, Austria). The clinicopathological characteristics in Table 1 are summarized as continuous variables and are expressed as n (%) or mean [standard deviation (SD)]. Categorical variables were analyzed using Pearson chi-squared test, and continuous variables test for normality. The continuous variables in Table 2 are nonnormally distributed and are expressed as mean (SD). MIE cohort and RAMIE cohort were taken as two independent sets of samples, and a nonparametric test (Mann-Whitney) was used to determine whether there were significant differences between these samples. The continuous variables in Table 3 are nonnormally distributed according to a Mann-Whitney test and are expressed as n (%) or mean [SD]. Statistical software SPSS and R were used to manage missing values via removal of cases. P values <0.05 were considered to be statistically significant.
Table 1
| Characteristic | All patients (N=270) | MIE (N=168) | RAMIE (N=102) | P value |
|---|---|---|---|---|
| Gender | 0.909 | |||
| Male | 263 (97.4) | 163 (97.0) | 100 (98.0) | |
| Female | 7 (2.6) | 5 (3.0) | 2 (2.0) | |
| Age, years | 59.98 [7.10] | 59.49 [7.27] | 60.77 [6.75] | 0.151 |
| BMI, kg/m2 | 23.19 [3.11] | 23.25 [3.03] | 23.09 [3.24] | 0.672 |
| Smoking | 0.912 | |||
| Never | 114 (42.2) | 70 (41.7) | 44 (43.1) | |
| Ever | 156 (57.8) | 98 (58.3) | 58 (56.9) | |
| Alcohol | 0.129 | |||
| Never | 110 (40.7) | 62 (36.9) | 48 (47.1) | |
| Ever | 160 (59.3) | 106 (63.1) | 54 (52.9) | |
| Tumor location | 0.487 | |||
| Ut | 38 (14.1) | 24 (14.3) | 14 (13.7) | |
| Mt | 92 (34.1) | 57 (33.9) | 35 (34.3) | |
| Lt | 136 (50.4) | 86 (51.2) | 50 (49.0) | |
| EGJ | 4 (1.5) | 1 (0.6) | 3 (2.9) | |
| Diabetes | <0.001* | |||
| No | 224 (83.0) | 151 (89.9) | 73 (71.6) | |
| Yes | 46 (17.0) | 17 (10.1) | 29 (28.4) | |
| Hypertension | 0.841 | |||
| No | 206 (76.3) | 127 (75.6) | 79 (77.5) | |
| Yes | 64 (23.7) | 41 (24.4) | 23 (22.5) | |
| Neoadjuvant therapy regimen | <0.001* | |||
| No | 150 (55.6) | 111 (66.1) | 39 (38.2) | |
| NC | 41 (15.2) | 28 (16.7) | 13 (12.7) | |
| NCI | 79 (29.3) | 29 (17.3) | 50 (49.0) | |
| Extent of lymph node dissection | 0.003* | |||
| Unknown | 1 (0.4) | 1 (0.6) | 0 (0.0) | |
| Three fields | 28 (10.4) | 12 (7.1) | 16 (15.7) | |
| Two fields | 147 (54.4) | 84 (50.0) | 63 (61.8) | |
| Two and a half fields | 94 (34.8) | 71 (42.3) | 23 (22.5) | |
| p-stage (UICC TNM 8th) | 0.023* | |||
| 0 | 5 (1.9) | 4 (2.4) | 1 (1.0) | |
| I | 99 (37.1) | 64 (38.8) | 35 (34.3) | |
| II | 72 (27.0) | 52 (31.5) | 20 (19.6) | |
| III | 82 (30.7) | 42 (25.5) | 40 (39.2) | |
| IVa | 9 (3.4) | 3 (1.8) | 6 (5.9) |
Data are presented as n (%) or mean [SD]. *, P values less than 0.05. Three cases in the MIE cohort had postoperative pathologic T-stage that could not be evaluated and were treated as missing values. MIE, minimally invasive esophagectomy; RAMIE, robot-assisted minimally invasive esophagectomy, BMI, body mass index; Ut, upper thoracic esophagus (from the superior margin of the sternum to the tracheal bifurcation); Mt, middle thoracic esophagus (superior half between the tracheal bifurcation and the esophagogastric junction); Lt, lower thoracic esophagus (thoracic esophagus from the inferior half between the tracheal bifurcation and the esophagogastric junction); EGJ, esophagogastric junction (the midpoint of the tumor within 2 cm of the cardia); NC, neoadjuvant chemotherapy; NCI, neoadjuvant chemoimmunotherapy; p-stage, pathological-stage; UICC, International Union against Cancer; TNM, tumor-node-metastasis; SD, standard deviation.
Table 2
| Characteristic | All patients (N=270) | MIE (N=168) | RAMIE (N=102) | P value |
|---|---|---|---|---|
| 105 | 1.28 (1.59) | 1.24 (1.56) | 1.34 (1.63) | 0.609 |
| 106recL | 4.24 (3.17) | 4.11 (3.26) | 4.46 (3.01) | 0.235 |
| 106recR | 2.96 (2.63) | 2.80 (2.58) | 3.23 (2.70) | 0.149 |
| 106tbL | 1.42 (2.25) | 1.01 (2.08) | 2.11 (2.35) | <0.001* |
| 106tbR | 0.12 (0.56) | 0.07 (0.40) | 0.21 (0.75) | 0.062 |
| 106pre | 0.46 (1.91) | 0.19 (1.09) | 0.90 (2.72) | 0.011* |
| 107 | 5.35 (3.79) | 6.08 (4.09) | 4.15 (2.86) | <0.001* |
| 108 | 1.12 (1.57) | 1.22 (1.78) | 0.95 (1.14) | 0.962 |
| 109L | 1.19 (1.64) | 0.89 (1.56) | 1.69 (1.65) | <0.001* |
| 109R | 1.03 (1.83) | 0.55 (1.25) | 1.81 (2.32) | <0.001* |
| 110 | 1.25 (1.67) | 1.11 (1.65) | 1.48 (1.69) | 0.004* |
| 111 | 1.00 (1.49) | 0.73 (1.15) | 1.44 (1.85) | <0.001* |
| 112pul | 0.33 (0.94) | 0.35 (1.09) | 0.29 (0.61) | 0.276 |
| 112aoA | 0.05 (0.36) | 0.03 (0.28) | 0.09 (0.47) | 0.065 |
| Number of thoracic lymph nodes dissected | 22.80 (9.71) | 20.82 (9.45) | 26.07 (9.28) | <0.001* |
| Number of lymph node groups that underwent thoracic lymph node dissection | 6.04 (2.11) | 5.28 (1.94) | 7.29 (1.77) | <0.001* |
Data are presented as mean (SD). *, P values less than 0.05. 105, the upper thoracic periesophageal lymph nodes; 106recL, the left recurrent laryngeal nerve lymph nodes; 106recR, the right recurrent laryngeal nerve; 106tbL, the left tracheobronchial lymph nodes; 106tbR, the right tracheobronchial lymph nodes; 106pre, the paratracheal lymph nodes; 107, the sublongitudinal lymph nodes; 108, the midthoracic periesophageal lymph node; 109L, the left main bronchial lymph nodes; 109R, the right main bronchial lymph nodes; 110, the subthoracic periesophageal lymph nodes; 111, the supradiaphragmatic lymph nodes; 112pul, the inferior pulmonary ligament lymph nodes; 112aoA, the thoracic anterior aortic lymph nodes; MIE, minimally invasive esophagectomy; RAMIE, robot-assisted minimally invasive esophagectomy; SD, standard deviation.
Table 3
| Outcomes | All patients (N=270) | MIE (N=168) | RAMIE (N=102) | P value |
|---|---|---|---|---|
| Pleural effusion (CD ≥I) | 133 (49.3) | 90 (53.6) | 43 (42.2) | 0.069 |
| Pneumonia (CD ≥II) | 61 (22.6) | 33 (19.6) | 28 (27.5) | 0.137 |
| Respiratory failure (CD ≥II) | 12 (4.4) | 5 (3.0) | 7 (6.9) | 0.133 |
| Anastomotic leakage (CD ≥II) | 13 (4.8) | 10 (6.0) | 3 (2.9) | 0.262 |
| Vocal cord paralysis | 14 (5.2) | 8 (4.8) | 6 (5.9) | 0.687 |
| Unilateral vocal cord paralysis | 8 (3.0) | 5 (3.0) | 3 (2.9) | |
| Bilateral vocal cord paralysis | 6 (2.2) | 3 (1.8) | 3 (2.9) | |
| Chest drainage, days | 5.01 [2.86] | 5.25 [3.19] | 4.62 [2.17] | 0.089 |
| Length of postoperative hospital stay, days | 13.36 [8.29] | 13.10 [7.25] | 13.79 [9.76] | 0.605 |
| Postoperative transfer to ICU | 71 (26.3) | 47 (28.0) | 24 (23.5) | 0.508 |
| Length of ICU stay, days | 1.33 [4.37] | 1.43 [4.19] | 1.18 [4.66] | 0.285 |
| In-hospital mortality | 0 | 0 | 0 | >0.99 |
Data are presented as n (%) or mean [SD]. CD, Clavien-Dindo classifications: grade I was defined as any deviation from the normal postoperative course without the need for pharmacologic treatment or surgical, endoscopic, or radiologic intervention; grade II was defined as pharmacologic treatment with drugs; grade III was defined as surgical, endoscopic, or radiologic intervention; grade IV was defined as life-threatening complications that required ICU management; and grade V was defined as mortality. MIE, minimally invasive esophagectomy; RAMIE, robot-assisted minimally invasive esophagectomy; ICU, intensive care unit; SD, standard deviation.
Results
Clinicopathological characteristics
In total, 285 patients with ESCC who underwent McKeown esophagectomy via right thoracotomy with lymphadenectomy between January 2019 and April 2023 were screened. These patients surgically were operated upon by the same experienced operator. Of these 285 patients, 9 patients who did not have postoperative pathology data and 6 patients who were diagnosed with nonsquamous cell carcinoma were excluded from the study. Ultimately, 270 patients were enrolled in this study and were divided into two groups according to the thoracic approach: MIE group (n=168) or RAMIE (n=102).
Table 1 summarizes the characteristics of all patients. Most patients were male (97.4%). The mean age was 60 (SD 7.1) years. The proportion of those with diabetes was significantly higher in the RAMIE cohort (28.4%) than in the MIE cohort (10.1%) (P<0.001). The proportion of neoadjuvant chemotherapy in the RAMIE cohort versus the MIE cohort was 12.7% and 16.7%, respectively, and the proportion of neoadjuvant chemoimmunotherapy was 49% and 17.3%, respectively. The patients were staged according the International Union against Cancer tumor-node-metastasis (TNM) classification system (eighth edition) (12). According to the pathological staging of the ECs, 5 (1.9%), 99 (37.1%), 72 (27.0%), 82 (30.7%), and 9 (3.4%) patients were p-stages 0, I, II, III, and IV, respectively. The MIE cohort involved significantly more cervical lymph node clearance because it had a lower proportion of patients who underwent neoadjuvant therapy than did the RAMIE cohort.
Postoperative pathologic lymph node outcomes
The postoperative pathologic lymph node outcomes are shown in Table 2. By comparing MIE cohort with RAMIE cohort, the transthoracic approach with RAMIE yielded a greater total number of thoracic lymph nodes dissected (MIE: mean 20.82, SD 9.45; RAMIE: mean 26.07, SD 9.28; P<0.001) and a greater total number of lymph node groups that underwent thoracic lymph node dissection (MIE: mean 5.28, SD 1.94; RAMIE mean 7.29, SD 1.77; P<0.001). The number of regional lymph node resections in the RAMIE cohort was significantly higher than that in the MIE cohorts for the following regions: 106tbL (P<0.001), 106pre (P=0.011), 107 (P<0.001), 109L (P<0.001), 109R (P<0.001), 110 (P=0.004), and 111 (P<0.001). Compared to MIE, RAMIE had higher mean of 106recL (4.46 vs. 4.11; P=0.235) and 106recR (3.23 vs. 2.80; P=0.149) although these differences were not significant.
Postoperative outcomes
Postoperative complications were categorized using the CD classifications (13). Table 3 depicts the postoperative outcomes. In the RAMIE cohort, pleural effusion (CD ≥I; n=43, 42.2%) occurred less frequently compared to the MIE (90, 53.6%) group but not significantly so (P=0.069). Similarly, the mean chest drainage time was lower in the RAMIE cohort (mean 4.62 days, SD 2.17 days) than in MIE cohort (mean 5.25 days, SD 3.19 days) but not significantly so (P=0.089). Between the RAMIE cohort and MIE cohort, there was no significant difference in pneumonia (CD ≥II; 27.5% vs. 19.6%), respiratory failure (CD ≥II; 6.9% vs. 3%), anastomotic leakage (CD ≥II; 2.9% vs. 6%), vocal cord paralysis (5.9% vs. 4.8%), mean postoperative length of hospital stay (13.79 days vs. 13.1 days), postoperative transfer to the ICU (23.5% vs. 28%), or mean length of ICU stay (1.18 vs. 1.43 days).
Discussion
The last two decades have witnessed a significant increase in surgical innovations aimed at improving the care of patients, and multidisciplinary treatment strategies for EC have greatly advanced. The aim of this study was to investigate the possible advantages of RAMIE over MIE for thoracic lymph node dissection in terms of postoperative outcomes. The results suggest that RAMIE provides a more complete resection of the regional lymph nodes at a higher stage of the tumor, with no meaningful difference in short-term postoperative outcomes. We found that a significantly greater portion of patients in the RAMIE cohort (49.0%) underwent neoadjuvant therapy than did those in the MIE group (17.3%) (P<0.001). Neoadjuvant chemoimmunotherapy can yield a high pathological complete response (pCR) rate with acceptable safety (14) and can increase opportunity for surgery in patients with locally advanced EC.
In this study, we also examined the differences in lymph nodes dissected between RAMIE and MIE across various regions of the chest. Table 2 shows that both the total number of thoracic lymph nodes removed (MIE: mean 20.82, SD 9.45; RAMIE: mean 26.07, SD 9.28; P<0.001) and the total number of thoracic lymph nodes groups removed (MIE: mean 5.28, SD 1.94; RAMIE mean 7.29, SD 1.77; P<0.001) in RAMIE cohort were significantly higher than MIE cohort. However, there was no statistical difference in the short-term outcomes. The possible reason for this is that there was a higher level of postoperative pathological-stage in the RAMIE cohort compared with the MIE cohort. This means that the RAMIE group had more difficult surgeries on average than did the MIE group. Related studies have reported that RAMIE can reduce surgical trauma, thus leading to decrease pneumonia rate, delayed gastric emptying rate and ICU stay (9,10). Moreover, the respiratory complications might have been reduced if the patients had surgery performed by surgeons after completion of their learning curve (15,16). In order to show the benefit of RAMIE versus conventional MIE in a study, a large number of cases will be needed because the differences in postoperative complications will be more subtle compared to minimally invasive versus open surgery. This could also be the reason for the non-significant results of the comparison of postoperative complications in our study.
Several single-institution studies have reported short-term outcomes of RAMIE for EC in terms of lymph node dissection and postoperative complications that are acceptable and comparable to those of conventional MIE, and Yang et al. reports that robotic assistance may be uniquely advantageous in the resection of lymph nodes adjacent to the recurrent laryngeal nerve, and van der Sluis et al. reports that RAMIE was associated with a lower overall, pulmonary, cardiac and wound complication rate as well as a shorter hospital stay compared to open or hybrid approach (6,9,17). Surgical resection with radical lymphadenectomy remains a critical element in the treatment of EC (18). From the advantages of lymph node removal, the robot’s multiple joints allow for multi-angle flip operations and filtering of human hand tremors. Theoretically it would raise the upper limit of the surgeon’s skill. Therefore, several studies have focused on examining the extent of lymph node dissection (19-21). Among the regional lymph nodes in the chest, the resection of group 106 is the most critical because patients with advanced EC often have recurrent laryngeal nerve lymph node metastases. In our study, 78.1% of patients underwent lymph node dissection of the bilateral recurrent laryngeal nerves. A previous large-scale retrospective study reported there to be no survival benefit and higher morbidity after bilateral recurrent laryngeal nerve lymph node dissection for patients with cancer in the lower thoracic esophagus or for older adult and female patients (22). Therefore, selective lymph node dissection of the recurrent laryngeal nerve in elderly patients with lesions located in the lower thoracic segment is also an option. Subject to the limited sample size of a single center, combining data from multiple centers would also provide a valid reference. A meta-analysis of 17 with sample sizes ranging from 10 to 634 patients indicated that the incidence of upper mediastinal lymph node metastases in distal esophageal adenocarcinoma to be as high as 10% [interquartile range (IQR), 4.7–16.7%] (23). However, this meta-analysis mainly included patients with adenocarcinoma and did not separately report the outcomes of patients with SCC. In our study, the number of upper mediastinal regional lymph node resections in the RAMIE cohort was significantly higher than that in the MIE group (106tbL: P<0.001; 106pre: P=0.011). Moreover, the mean of 106recL (4.46 vs. 4.11; P=0.235) and 106recR (3.23 vs. 2.80; P=0.149) in the RAMIE cohort were higher than those than in the group MIE although these differences were not significant. But, at station 107, MIE (MIE: mean 6.08 vs. RAMIE: mean 4.15, P<0.001) appeared to remove more lymph nodes than RAMIE. This may also be related to the MIE cohort’s insufficiently fine anatomical manipulation of the subglottic lymph nodes and bilateral bronchial lymph nodes. The insufficiently fine manipulation did not allow a good distinction between 107 and 109, resulting in a difference in the number of 107 and 109 dissection between the two cohorts. Lymphatic channels within the esophagus are highly complex, resulting in a variable lymphatic spread and skip metastases, standard two-field lymph node dissection is necessary in patients with EC (24). A randomized multicenter study reported that a higher number of lymph nodes dissected is associated with improved survival and local disease control. Therefore, systemic lymphadenectomy should still be considered an integral part of surgical resection even after neoadjuvant chemoradiotherapy for locally advanced ESCC (25). Advances in surgical techniques have also increased the number of lymph nodes that can be removed (26). In one study, a robotic approach to esophagectomy was associated with a lower likelihood of unplanned conversion to open surgery, and patients who were converted to open surgery experienced worse outcomes (27). RAMIE is a safe and feasible alternative to MIE and may be superior in lymph node dissection of the abdominal cavity and the left recurrent laryngeal nerve (21). By comparing MIE cohort with RAMIE cohort, our research also shows that the transthoracic approach in RAMIE yielded a greater total number of thoracic lymph nodes dissected (MIE: mean 20.82, SD 9.45; RAMIE: mean 26.07, SD 9.28; P<0.001) and a greater number of total of lymph node groups that underwent thoracic lymph node dissection (MIE: mean 5.28, SD 1.94; RAMIE: mean 7.29, SD 1.77; P<0.001).
Neoadjuvant chemoimmunotherapy plus RAMIE involves higher healthcare costs, and this combination is particularly expensive in China. Postoperative complications are an important factor affecting healthcare costs of postoperative hospitalization (28). In our study, RAMIE and MIE showed no significant difference in pneumonia (CD ≥II; 27.5% vs. 19.6%), respiratory failure (CD ≥II; 6.9% vs. 3.0%), anastomotic leakage (CD ≥II; 2.9% vs. 6.0%), vocal cord paralysis (5.9% vs. 4.8%), mean length of postoperative hospital stay (13.79 vs. 13.10 days), postoperative transfer to the ICU (23.5% vs. 28.0%), and mean length of ICU stay (1.18 vs. 1.43 days). In a national cohort study in the United States that directly compared MIE to RAMIE, there was no difference in the rate of pulmonary complications, anastomotic leak, all-cause morbidity, or mortality (29). In a 7-year National Cancer Database cohort study, overall survival was significantly longer in patients with EC who underwent RAMIE than in those who underwent MIE (30). However, the long-term survival outcomes of patients of ESCC in our study need to be supported by more extensive follow-up data.
There are several limitations to this study. First, we employed a retrospective, single-center design that was susceptible to selection bias. Still, we also tried to partially compensate for this bias by making a large effort to expand the sample size. Second, a single surgeon operation may reduce baseline bias. It is worth noting that it is not possible to perform unchanging preoperative preparation and postoperative management of patients with individuality. Third, the follow-up of the patients in the RAMIE cohort was relatively short, and therefore no conclusions could be drawn concerning disease-free survival or overall survival. Fourth, both the RAMIE and MIE cases were selected from January 2019 through April 2023. Both approaches have been in use by surgeons during this period. The RAMIE cases included cases from other clinical trial groups regarding the use of robotic platform, as well as patients who were not included. Therefore, the criteria have not been fully standardized with regard to the choice of surgical approach.
Conclusions
Our study shows that RAMIE may be more effective than MIE in terms of the number of thoracic lymph nodes dissected and the extent of dissection. Moreover, RAMIE may be not associated with additional surgical complications.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-201/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-201/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-201/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-201/coif). J.M. serves as an unpaid editorial board member of Journal of Thoracic Disease from December 2023 to November 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Harbin Medical University Cancer Hospital (No. 2020-50-IIT), and all enrolled patients consented to the use of their clinical data by signing informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Waters JK, Reznik SI. Update on Management of Squamous Cell Esophageal Cancer. Curr Oncol Rep 2022;24:375-85. [Crossref] [PubMed]
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- Kelly RJ. Emerging Multimodality Approaches to Treat Localized Esophageal Cancer. J Natl Compr Canc Netw 2019;17:1009-14. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection. Annals of Surgery 2017;266:232-6. [Crossref] [PubMed]
- Yang Y, Li B, Yi J, et al. Robot-assisted Versus Conventional Minimally Invasive Esophagectomy for Resectable Esophageal Squamous Cell Carcinoma. Annals of Surgery 2022;275:646-53. [Crossref] [PubMed]
- Mann C, Jezycki T, Berlth F, et al. Effect of thoracic cage width on surgery time and postoperative outcome in minimally invasive esophagectomy. Surg Endosc 2023;37:8301-8. [Crossref] [PubMed]
- Weindelmayer J, De Pasqual CA, Turolo C, et al. Robotic versus open Ivor-Lewis esophagectomy: A more accurate lymph node dissection without burdening the leak rate. J Surg Oncol 2023;127:1109-15. [Crossref] [PubMed]
- van der Sluis PC, Babic B, Uzun E, et al. Robot-assisted and conventional minimally invasive esophagectomy are associated with better postoperative results compared to hybrid and open transthoracic esophagectomy. Eur J Surg Oncol 2022;48:776-82. [Crossref] [PubMed]
- van der Sluis PC, Schizas D, Liakakos T, et al. Minimally Invasive Esophagectomy. Dig Surg 2020;37:93-100. [Crossref] [PubMed]
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. Journal of Thoracic Oncology 2017;12:36-42.
- Dindo D, Demartines N, Clavien P-A. Classification of Surgical Complications. Annals of Surgery 2004;240:205-13. [Crossref] [PubMed]
- Ozawa S, Uchi Y, Ando T, et al. Essential updates 2020/2021: Recent topics in surgery and perioperative therapy for esophageal cancer. Ann Gastroenterol Surg 2023;7:346-57. [Crossref] [PubMed]
- Rebecchi F, Ugliono E, Allaix ME, et al. Why pay more for robot in esophageal cancer surgery? Updates Surg 2023;75:367-72. [Crossref] [PubMed]
- Chan KS, Oo AM. Exploring the learning curve in minimally invasive esophagectomy: a systematic review. Dis Esophagus 2023;36:doad008. [Crossref] [PubMed]
- Trung LV, Loc NVV, Tien TPD, et al. Robot-Assisted Versus Thoraco-laparoscopic McKeown Esophagectomy for Esophageal Cancer: a Propensity Score-Matched Study. J Gastrointest Surg 2022;26:1093-6. [Crossref] [PubMed]
- Yang Y, Zhang X, Li B, et al. Robot-assisted esophagectomy (RAE) versus conventional minimally invasive esophagectomy (MIE) for resectable esophageal squamous cell carcinoma: protocol for a multicenter prospective randomized controlled trial (RAMIE trial, robot-assisted minimally invasive Esophagectomy). BMC Cancer 2019;19:608. [Crossref] [PubMed]
- Liang R, Bi X, Fan D, et al. Mapping of lymph node dissection determined by the epicenter location and tumor extension for esophagogastric junction carcinoma. Front Oncol 2022;12:913960. [Crossref] [PubMed]
- Kurokawa Y, Takeuchi H, Doki Y, et al. Mapping of Lymph Node Metastasis From Esophagogastric Junction Tumors. Annals of Surgery 2021;274:120-7. [Crossref] [PubMed]
- Zhang Y, Dong D, Cao Y, et al. Robotic Versus Conventional Minimally Invasive Esophagectomy for Esophageal Cancer. Annals of Surgery 2023;278:39-50. [Crossref] [PubMed]
- Zhang S, Liu Q, Li B, et al. Clinical significance and outcomes of bilateral and unilateral recurrent laryngeal nerve lymph node dissection in esophageal squamous cell carcinoma: A large-scale retrospective cohort study. Cancer Medicine 2022;11:1617-29. [Crossref] [PubMed]
- Gantxegi A, Kingma BF, Ruurda JP, et al. The Value of Paratracheal Lymphadenectomy in Esophagectomy for Adenocarcinoma of the Esophagus or Gastroesophageal Junction: A Systematic Review of the Literature. Ann Surg Oncol 2022;29:1347-56. [Crossref] [PubMed]
- Ji X, Cai J, Chen Y, et al. Lymphatic spreading and lymphadenectomy for esophageal carcinoma. World J Gastrointest Surg 2016;8:90-4. [Crossref] [PubMed]
- Guo X, Wang Z, Yang H, et al. Impact of Lymph Node Dissection on Survival After Neoadjuvant Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Carcinoma. Annals of Surgery 2023;277:259-66. [Crossref] [PubMed]
- Chen WS, Zhu LH, Li WJ, et al. Novel technique for lymphadenectomy along left recurrent laryngeal nerve during thoracoscopic esophagectomy. World J Gastroenterol 2020;26:1340-51. [Crossref] [PubMed]
- Silva JP, Putnam LR, Wu J, et al. Lower Rates of Unplanned Conversion to Open in Robotic Approach to Esophagectomy for Cancer. Am Surg 2023;89:2583-94. [Crossref] [PubMed]
- Goense L, van der Sluis PC, van der Horst S, et al. Cost analysis of robot-assisted versus open transthoracic esophagectomy for resectable esophageal cancer. Results of the ROBOT randomized clinical trial. Eur J Surg Oncol 2023;49:106968. [Crossref] [PubMed]
- Turner KM, Delman AM, Johnson K, et al. Robotic-Assisted Minimally Invasive Esophagectomy: Postoperative Outcomes in a Nationwide Cohort. J Surg Res 2023;283:152-60. [Crossref] [PubMed]
- Byiringiro I, Aurit SJ, Nandipati KC. Long-term survival outcomes associated with robotic-assisted minimally invasive esophagectomy (RAMIE) for esophageal cancer. Surg Endosc 2023;37:4018-27. [Crossref] [PubMed]