
Efficacy and safety analysis of immune checkpoint inhibitor rechallenge therapy in locally advanced and advanced non-small cell lung cancer: a retrospective study
Highlight box
Key findings
• In clinical practice, immune checkpoint inhibitor (ICI) rechallenge therapy beyond disease progression may not improve clinical outcomes in locally advanced or advanced non-small cell lung cancer (NSCLC) patients, but patients with favorable response to initial ICIs treatment still showed significant efficacy of subsequent ICI rechallenge therapy.
What is known and what is new?
• The use of ICIs has revolutionized the treatment of NSCLC without driver gene alterations, achieved high response rates in first-line treatment. Majority of patients experience disease progression after initial treatment, the optimal choice for second-line treatment and the efficacy and safety of ICI rechallenge therapy are unclear.
• In our study, ICI rechallenge therapy beyond disease progression did not improve clinical outcomes in patients with NSCLC. There were no significant differences in objective response rate and progression-free survival (PFS) between the ICI rechallenge group and non-rechallenge group. However, patients responded to initial ICIs treatment had better PFS. In multivariate analysis, ICI rechallenge therapy and combination of radiotherapy during the second-line treatment were not independent predictors of PFS.
What is the implication, and what should change now?
• Further studies should examine efficacy and safety of different second-line treatment regimens in large randomized prospective cohorts, and determine which patient groups can easily benefit from ICI rechallenge therapy.
Introduction
Lung cancer is the most common cancer worldwide as well as the leading cause of cancer related death with an estimated 1.8 million deaths each year (1). Non-small cell lung cancer (NSCLC) accounts for up to 85% of all lung cancers (2). Most patients are at an unresectable locally advanced or an advanced stage at the time of initial diagnosis, with a very low 5-year survival rate (3). In recent years, immune checkpoint inhibitors (ICIs), especially programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors, has become the first-line standard of care for NSCLC patients without driver gene alterations because of the remarkable efficacy and tolerable adverse effects (4,5). Despite these advances, the majority of patients experience disease progression after initial treatment. To date, the mechanisms of resistance to first-line immunotherapy are not clear (6). The optimal choice for second-line treatment and the efficacy and safety of ICI rechallenge therapy are currently unknown.
Current treatment guidelines recommend monotherapy as second-line treatment whenever possible. Since disease progression is often associated with drug tolerance, guidelines recommend shifting to a different drug in the second-line treatment after disease progression. However, due to limited efficacy of monotherapy, clinicians are still focused on multiple combination regimens to overcome the ICIs resistance. PD-1/PD-L1 inhibitors are recommended as second-line regimen for locally advanced and advanced NSCLC, including squamous and non-squamous cancers. Several ICIs have shown significant efficacy in the second-line treatment of NSCLC without driver gene alterations (7-9). One study has shown clinically meaningful efficacy of ICI rechallenge therapy without increasing toxicity (10), which suggested that patients who respond to initial ICIs treatment are likely to respond to ICI rechallenge. Although analysis of ICIs treatment beyond progression of anti-PD-1/PD-L1 in melanoma has been reported in one study (11), there are fewer studies of NSCLC. Therefore, there are no precise conclusions as to whether ICI rechallenge therapy is effective and safe in the second-line treatment for locally advanced and advanced NSCLC patients.
Previous studies have confirmed that antiangiogenic agents have the potential to modulate the tumor microenvironment and improve cancer immunotherapy (12,13). There are many interactions between angiogenesis and immune escape (14). The Impower150 study showed that the addition of atezolizumab to bevacizumab plus chemotherapy significantly improved progression-free survival and overall survival (OS) among patients with metastatic non-squamous NSCLC (15). The results of several recent large studies have demonstrated that antiangiogenic drugs in combination with other therapeutic regimens may be able to reverse PD-1/PD-L1 inhibitors resistance with favorable efficacy and safety (16-18). This raises the question of whether antiangiogenic drugs can be used as combination regimens for second-line therapy in locally advanced and advanced NSCLC.
The management of NSCLC patients who experienced disease progression at the end of the first-line treatment remains a clinical challenge. The selection of appropriate second-line treatment strategies is critical in order to effectively manage disease progression and improve clinical outcomes. The aim of this retrospective study was to assess the efficacy and safety of ICI rechallenge therapy in locally advanced or advanced NSCLC patients. In addition, we aimed to determine which patient groups would benefit from ICI rechallenge therapy and provide a reference for clinical decision-making. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1767/rc).
Methods
Patients
We retrospectively reviewed the medical records of locally advanced or advanced NSCLC patients who received treatment at the Jiangsu Cancer Hospital between January 2019 and June 2022. The last follow-up and data collection were updated as of May 2023. The inclusion criteria were as follows: (I) patients aged 18–80 years; (II) patients who scored 0–2 on the Eastern Cooperative Oncology Group performance status (ECOG PS); (III) patients without driver gene alterations; (IV) patients histologically or cytologically diagnosed with unresectable stage III/IV NSCLC; (V) patients who had received the PD-1/PD-L1 inhibitors alone or in combination with the chemotherapy and/or antiangiogenic drugs in the first-line treatment and defined progress disease (PD) at the end of the first line; (VI) complete clinicopathological data for evaluation. All patients included in this study had at least one measurable disease. The ICIs used in the study included pembrolizumab, nivolumab, camrelizumab, toripalimab, tislelizumab and sintilimab. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Academic Ethics Committee of Jiangsu Cancer Hospital [No. (2023)082], and individual consent for this retrospective analysis was waived.
Data collection and response assessment
Medical records were collected and extracted on clinical pathologic features and treatment histories. Data and follow-up records were updated as of May 2023. The best response, defined as a complete response (CR) or partial response (PR) and stable disease (SD) achieved at least once during the process of therapy, was assessed using the RECIST v1.1 criteria. The objective response rate (ORR) was defined as the proportion of patients with the best overall response of CR or PR among all patients, and the disease control rate (DCR) was defined as the proportion of patients with the best overall response of CR or PR or SD. Progression-free survival 1 (PFS1) was defined as the time of initiation of PD-1/PD-L1 inhibitors alone or in combination with the chemotherapy and/or antiangiogenic drugs to first defined PD. Progression-free survival 2 (PFS2) was the time from the first defined PD to the second disease progression or death. The resistant group was defined as patients with PFS1 of less than six months, and the responder group was defined as patients with PFS1 of longer than six months. Adverse events (AEs) were recorded and graded by the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0.
Statistical analysis
Survival data were estimated using the Kaplan-Meier method and the log-rank test was used to assess between-group differences in progression-free survival. Hazard ratios (HRs) and associated 95% confidence interval (CI) were calculated with the use of the Cox proportional-hazards model. Chi-squared or Fisher’s exact test was used to compare the differences in baseline between different groups. Statistical analyses were conducted using the SPSS version 26.0 (SPSS, Inc., Chicago, IL, USA). P≤0.05 was considered to indicate statistical significance.
Results
Patients’ characteristics
In total, 224 patients were enrolled in this study. Their clinical and pathological baseline characteristics are shown in Table 1. The median age of the 224 patients was 65 years, and our sample included 188 males and 36 females. The majority of patients (53.1%, 119/224) was diagnosed with lung adenocarcinoma, followed by squamous cell carcinoma (43.3%). Most (75.0%) patients were clinically diagnosed with stage IV lung cancer and most patients (75.0%) did not receive combined radiotherapy during the second-line treatment. Most (85.3%) patients had an ECOG PS of 0 or 1. All patients had received PD-1/PD-L1 inhibitors alone or in combination with chemotherapy and/or antiangiogenic drugs during the first-line treatment and were finally evaluated as PD in first-line treatment.
Table 1
| Characteristic | Patients, n (%) | CR | PR | SD | PD | ORR (%) | Hypothesis testing parameters of ORR | DCR (%) | Hypothesis testing parameters of DCR |
|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||
| Female | 36 (16.1) | 0 | 5 | 20 | 11 | 13.9 | 0.855 | 69.4 | 0.558 |
| Male | 188 (83.9) | 0 | 21 | 100 | 67 | 11.2 | 64.4 | ||
| Age, years | |||||||||
| ≤60 | 81 (36.2) | 0 | 9 | 40 | 32 | 11.1 | 0.862 | 60.5 | 0.268 |
| >60 | 143 (63.8) | 0 | 17 | 80 | 46 | 11.9 | 67.8 | ||
| Smoking status | |||||||||
| Current or former | 55 (24.6) | 0 | 5 | 32 | 18 | 9.1 | 0.502 | 67.3 | 0.707 |
| Never | 169 (75.4) | 0 | 21 | 88 | 60 | 12.4 | 64.5 | ||
| Histology | |||||||||
| Adenocarcinoma | 119 (53.1) | 0 | 16 | 68 | 35 | 13.4 | 0.172 | 70.6 | 0.165 |
| Squamous | 97 (43.3) | 0 | 8 | 49 | 40 | 8.2 | 58.8 | ||
| Other | 8 (3.6) | 0 | 2 | 3 | 3 | 25.0 | 62.5 | ||
| Stage of cancer | |||||||||
| Stage III | 56 (25.0) | 0 | 3 | 36 | 17 | 5.4 | 0.092 | 69.6 | 0.418 |
| Stage IV | 168 (75.0) | 0 | 23 | 84 | 61 | 13.7 | 63.7 | ||
| Combined with radiotherapy | |||||||||
| Yes | 56 (25.0) | 0 | 12 | 32 | 12 | 21.4 | 0.008 | 78.6 | 0.015 |
| No | 168 (75.0) | 0 | 14 | 88 | 66 | 8.3 | 60.7 | ||
| ECOG PS | |||||||||
| 0 | 46 (20.5) | 0 | 7 | 26 | 13 | 15.2 | 0.680 | 71.7 | 0.032 |
| 1 | 145 (64.7) | 0 | 16 | 82 | 47 | 11.0 | 67.6 | ||
| 2 | 33 (14.7) | 0 | 3 | 12 | 18 | 9.1 | 45.5 | ||
| Second-line treatment received | |||||||||
| Chemotherapy alone | 21 (9.4) | 0 | 1 | 9 | 11 | 4.8 | 0.199 | 47.6 | 0.025 |
| C + A | 38 (17.0) | 0 | 8 | 18 | 12 | 21.1 | 68.4 | ||
| ICI monotherapy | 5 (2.2) | 0 | 0 | 3 | 2 | 0.0 | 60.0 | ||
| ICIs + C | 77 (34.4) | 0 | 5 | 40 | 32 | 6.5 | 58.4 | ||
| ICIs + C + A | 51 (22.8) | 0 | 8 | 26 | 17 | 15.7 | 66.7 | ||
| ICIs + A | 32 (14.3) | 0 | 4 | 24 | 4 | 12.5 | 87.5 | ||
| Total | 224 | 0 | 26 | 120 | 78 | 11.6 | 65.2 | ||
CR, complete response; PR, partial response; SD, stable disease; PD, progress disease; ORR, objective response rate; DCR, disease control rate; ECOG PS, Eastern Cooperative Oncology Group performance status; C + A, chemotherapy with antiangiogenic drugs; ICI, immune checkpoint inhibitor; ICIs + C, immune checkpoint inhibitors with chemotherapy; ICIs + C + A, immune checkpoint inhibitors with chemotherapy and antiangiogenic drugs; ICIs + A, immune checkpoint inhibitors with antiangiogenic drugs.
Treatment characteristics
As shown in Table 1, 77 patients (34.4%) received ICIs combined with chemotherapy (ICIs + C) as second-line therapy, 51 patients (22.8%) received ICIs combined with chemotherapy and antiangiogenic drugs (ICIs + C + A), 38 patients (17.0%) received chemotherapy combined with antiangiogenic drugs (C + A), 32 patients (14.3%) received ICIs combined with antiangiogenic drugs (ICIs + A), 21 patients (9.4%) received chemotherapy alone and five patients (2.2%) received ICI monotherapy treatment. In addition, some (25%, 56/224) patients received radiotherapy during the second-line treatment. Among the 224 patients, 165 (73.7%) patients had an anti-PD-1 or anti-PD-L1 rechallenge in the second-line treatment. In ICI rechallenge group, 51 patients resistant to first-line immunotherapy were included in resistant group. And 114 patients responded to first-line immunotherapy were included in responder group.
Overall clinical outcomes
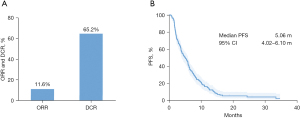
As shown in Figure 1A, the ORR was observed in 26 of 224 (11.6%) patients, and the DCR was 65.2% (146/224). No patient achieved a CR. The mPFS2 was 5.06 months with 95% CI of 4.02–6.10 months (Figure 1B). Twelve of the 26 patients with PR were treated with radiotherapy combined with the second-line therapy. Among those 26 patients who achieved a PR, 25 were treated with combination regimens and only one had the monotherapy, but the result showed no statistically significant difference (P=0.323).
Subgroup analyses
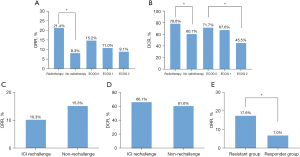
The ORR, DCR in radiotherapy group were significantly higher and longer than in the no radiotherapy group (ORR: 21.4% vs. 8.3%, P=0.008, Figure 2A; DCR: 78.6% vs. 60.7%, P=0.015, Figure 2B). Five patients received the PD-1/PD-L1 inhibitor monotherapy in the second-line treatment, none of them achieved complete or PR. Patients with better ECOG PS had higher DCR than those with poorer performance status (DCR: 71.7% vs. 67.6% vs. 45.5%, P=0.032, Figure 2B). ORR for the second-line treatment was numerically higher in the C + A group (21.1%, P=0.199) and DCR was the highest in the ICIs + A group (87.5%, P=0.025). ORR and DCR were similar between the ICI rechallenge therapy group and non-rechallenge group (ORR: 10.3% vs. 15.3%, P=0.308, Figure 2C; DCR: 66.7% vs. 61.0%, P=0.434, Figure 2D). In ICI rechallenge group, the ORR was significantly higher in the responder group than in the resistant group (ORR: 17.6% vs. 7.0%, P=0.038, Figure 2E).
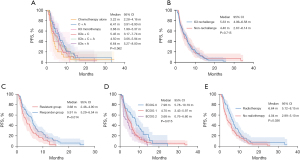
In the second-line treatment, mPFS2 was 6.64 months (95% CI: 5.27–8.00) in the ICIs + A group; 6.41 months (95% CI: 3.91–8.90) in the C + A group; 5.45 months (95% CI: 3.17–7.74) in the ICIs + C group; 4.50 months (95% CI: 3.06–5.94) in the ICIs + C + A group; 3.68 months (95% CI: 1.99–5.37) in the ICI monotherapy group and 3.22 months (95% CI: 2.28–4.16) in the chemotherapy alone group (Figure 3A, P=0.362). However, no statistical difference in efficacy was observed possibly because of the limited number of patients. The mPFS2 was 5.33 months (95% CI: 4.06–6.58) in ICI rechallenge therapy group and 4.40 months (95% CI: 2.67–6.14) in non-rechallenge group (HR =1.062, 95% CI: 0.77–1.47; P=0.715, Figure 3B). In ICI rechallenge group, PFS2 was significantly longer in the responder group than in the resistant group (mPFS2: 5.91 vs. 3.68 months, P=0.014, Figure 3C). Patients with better ECOG PS had longer PFS2 than those with poorer performance status (mPFS2: 7.98 vs. 4.70 vs. 3.68 months, P=0.015, Figure 3D). PFS2 in radiotherapy group was significantly longer than in the no radiotherapy group (mPFS2: 6.64 vs. 4.04 months, P=0.036, Figure 3E).
Multivariate Cox regression analysis of all patients (n=224), including sex, age, smoking status, histology, ECOG PS score, tumor, node, metastasis (TNM) stage, combination of radiotherapy and therapeutic regimen, further confirmed that ICI rechallenge therapy and combination of radiotherapy during the second-line treatment were not independent prognostic factors affecting PFS2 (Table 2). As shown in Table 3, multivariate analysis of the ICI rechallenge group (n=165) showed that response to initial ICIs treatment was associated with PFS2 (P=0.015).
Table 2
| Variable | Category | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| Sex | Male vs. female | 0.939 (0.634–1.391) | 0.754 | |||
| Age | >60 vs. ≤60 years | 0.926 (0.687–1.249) | 0.615 | |||
| Smoking status | Smoker vs. never smoker | 0.936 (0.666–1.314) | 0.701 | |||
| Histology | Non-adenocarcinoma vs. adenocarcinoma | 1.109 (0.829–1.484) | 0.485 | |||
| TNM stage | IV vs. III | 1.323 (0.936–1.872) | 0.113 | |||
| Combination of radiotherapy | Radiotherapy vs. no radiotherapy | 0.702 (0.503–0.979) | 0.037 | |||
| ECOG PS | ≥1 vs. 0 | 1.595 (1.100–2.313) | 0.014 | 1.518 (1.044–2.207) | 0.029 | |
| Therapeutic regimen | Non-rechallenge vs. ICI rechallenge | 0.941 (0.680–1.302) | 0.715 | |||
HR, hazard ratio; CI, confidence interval; TNM, tumor, node, metastasis; ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor.
Table 3
| Variable | Category | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| Sex | Male vs. female | 0.999 (0.636–1.570) | 0.998 | |||
| Age | >60 vs. ≤60 years | 0.997 (0.698–1.425) | 0.987 | |||
| Smoking status | Smoker vs. never smoker | 0.888 (0.605–1.304) | 0.545 | |||
| Histology | Non-adenocarcinoma vs. adenocarcinoma | 1.127 (0.801–1.587) | 0.492 | |||
| TNM stage | IV vs. III | 1.456 (0.942–2.249) | 0.091 | |||
| Combination of radiotherapy | Radiotherapy vs. no radiotherapy | 0.798 (0.541–1.179) | 0.257 | |||
| ECOG PS | ≥1 vs. 0 | 2.005 (1.295–3.104) | 0.002 | 2.006 (1.295–3.108) | 0.002 | |
| Response to initial ICIs treatment | Responder group vs. resistant group | 0.640 (0.447–0.916) | 0.015 | 0.640 (0.447–0.917) | 0.015 | |
ICI, immune checkpoint inhibitor; HR, hazard ratio; CI, confidence interval; TNM, tumor, node, metastasis; ECOG PS, Eastern Cooperative Oncology Group performance status.
Safety
As shown in Table 4, 65.2% (146/224) of patients experienced treatment-related AEs (TRAEs) in the second-line treatment. Several (17.0%, 38/224) patients experienced grade 3 or 4 AEs. No grade 5 TRAE was reported. The number of patients with TRAEs was 47 (61.0%), 24 (63.2%), 19 (59.4%), 35 (68.6%), 3 (60.0%) and 18 (85.7%) in the ICIs + C, C + A, ICIs + A, ICIs + C + A, ICI monotherapy and chemotherapy alone groups, respectively, while the incidence of grade 3 or 4 TRAEs in the same groups was 13.0%, 18.4%, 6.3%, 23.5%, 20.0%, and 28.6%, respectively. Similar incidences of TRAEs at any grade were observed in the different second-line treatment groups (P=0.334). Similarly, no statistically significant differences in the incidence of grade 3 and 4 AEs were observed (P=0.162).
Table 4
| Events | Grade | ICIs + C (N=77) |
ICIs + C + A (N=51) | ICIs + A (N=32) |
C + A (N=38) |
ICI monotherapy (N=5) | Chemotherapy alone (N=21) |
|---|---|---|---|---|---|---|---|
| Hypothyroidism | Any grade | 2 (2.6) | 5 (9.8) | 4 (12.5) | 3 (7.9) | 1 (20.0) | 0 |
| Grade 3–5 | 0 | 0 | 0 | 1 (2.6) | 0 | 0 | |
| Neutropenia | Any grade | 16 (20.8) | 10 (19.6) | 3 (9.4) | 4 (10.5) | 0 | 3 (14.3) |
| Grade 3–5 | 5 (6.5) | 5 (9.8) | 0 | 3 (7.9) | 0 | 2 (9.5) | |
| Pneumonia | Any grade | 2 (2.6) | 4 (7.8) | 0 | 1 (2.6) | 1 (20.0) | 0 |
| Grade 3–5 | 0 | 2 (3.9) | 0 | 0 | 1 (20.0) | 0 | |
| Anemia | Any grade | 25 (32.5) | 18 (35.3) | 11 (34.4) | 17 (44.7) | 1 (20.0) | 14 (66.7) |
| Grade 3–5 | 2 (2.6) | 5 (9.8) | 0 | 2 (5.3) | 0 | 3 (14.3) | |
| Arrhythmia | Any grade | 1 (1.3) | 5 (9.8) | 0 | 2 (5.3) | 0 | 2 (9.5) |
| Grade 3–5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Thrombocytopenia | Any grade | 11 (14.3) | 12 (23.5) | 4 (12.5) | 5 (13.2) | 0 | 6 (28.6) |
| Grade 3–5 | 3 (3.9) | 4 (7.8) | 0 | 2 (5.3) | 0 | 3 (14.3) | |
| Leukopenia | Any grade | 19 (24.7) | 15 (29.4) | 5 (15.6) | 5 (13.2) | 0 | 5 (23.8) |
| Grade 3–5 | 3 (3.9) | 3 (5.9) | 0 | 3 (7.9) | 0 | 3 (14.3) | |
| ALT/AST elevated | Any grade | 8 (10.4) | 8 (15.7) | 2 (6.3) | 4 (10.5) | 1 (20.0) | 4 (19.0) |
| Grade 3–5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nasal bleeding | Any grade | 0 | 1 (2.0) | 0 | 1 (2.6) | 0 | 0 |
| Grade 3–5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nausea | Any grade | 2 (2.6) | 4 (7.8) | 0 | 0 | 0 | 1 (4.8) |
| Grade 3–5 | 0 | 1 (2.0) | 0 | 0 | 0 | 2 (9.5) | |
| Joint pain | Any grade | 0 | 1 (2.0) | 0 | 1 (2.6) | 0 | 0 |
| Grade 3–5 | 0 | 1 (2.0) | 0 | 0 | 0 | 0 | |
| Rash | Any grade | 3 (3.9) | 3 (5.9) | 0 | 0 | 0 | 0 |
| Grade 3–5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Diarrhea | Any grade | 0 | 2 (3.9) | 2 (6.3) | 0 | 0 | 1 (4.8) |
| Grade 3–5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hair loss | Any grade | 1 (1.3) | 1 (2.0) | 0 | 0 | 0 | 0 |
| Grade 3–5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Poor appetite | Any grade | 1 (1.3) | 2 (3.9) | 1 (3.1) | 0 | 0 | 1 (4.8) |
| Grade 3–5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Fatigue | Any grade | 2 (2.6) | 2 (3.9) | 1 (3.1) | 0 | 0 | 1 (4.8) |
| Grade 3–5 | 1 (1.3) | 0 | 1 (3.1) | 0 | 0 | 0 | |
| Bilirubin increased | Any grade | 2 (2.6) | 1 (2.0) | 1 (3.1) | 0 | 0 | 0 |
| Grade 3–5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Creatinine increased | Any grade | 5 (6.5) | 0 | 4 (12.5) | 2 (5.3) | 1 (20.0) | 2 (9.5) |
| Grade 3–5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Proteinuria | Any grade | 1 (1.3) | 0 | 0 | 0 | 0 | 0 |
| Grade 3–5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Fever | Any grade | 2 (2.6) | 0 | 1 (3.1) | 2 (5.3) | 0 | 1 (4.8) |
| Grade 3–5 | 1 (1.3) | 0 | 1 (3.1) | 1 (2.6) | 0 | 1 (4.8) | |
| Paresthesia | Any grade | 1 (1.3) | 0 | 0 | 1 (2.6) | 0 | 0 |
| Grade 3–5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hyperuricemia | Any grade | 0 | 0 | 0 | 1 (2.6) | 0 | 0 |
| Grade 3–5 | 0 | 0 | 0 | 1 (2.6) | 0 | 0 |
Data are presented as n (%). ICIs + C, immune checkpoint inhibitors with chemotherapy; ICIs + C + A, immune checkpoint inhibitors with chemotherapy and antiangiogenic drugs; ICIs + A, immune checkpoint inhibitors with antiangiogenic drugs; C + A, chemotherapy with antiangiogenic drugs; ICI, immune checkpoint inhibitor; ALT, alanine aminotransferase; AST, aspartate transaminase.
The detailed AEs in ICI rechallenge group and non-rechallenge group are presented in Table 5. Similar incidences of TRAEs at any grade were observed in ICI rechallenge group and non-rechallenge group (63.0% vs. 71.2%, P=0.259), and no statistically significant differences in the incidence of grade 3 and 4 AEs were observed between the two groups (15.2% vs. 22.0%, P=0.227). Similar incidences of TRAEs at any grade were observed in the resistant group and responder group (68.6% vs. 60.5%, P=0.319), and no statistically significant differences in the incidence of grade 3 and 4 AEs were observed between the two groups (17.6% vs. 14.0%, P=0.550) (Table 6).
Table 5
| Events | Grade | ICI rechallenge (N=165) | Non-rechallenge (N=59) |
|---|---|---|---|
| Hypothyroidism | Any grade | 12 (7.3) | 3 (5.1) |
| Grade 3–5 | 0 | 1 (1.7) | |
| Neutropenia | Any grade | 29 (17.6) | 7 (11.9) |
| Grade 3–5 | 10 (6.1) | 5 (8.5) | |
| Pneumonia | Any grade | 7 (4.2) | 1 (1.7) |
| Grade 3–5 | 3 (1.8) | 0 | |
| Anemia | Any grade | 55 (33.3) | 31 (52.5) |
| Grade 3–5 | 7 (4.2) | 5 (8.5) | |
| Arrhythmia | Any grade | 6 (3.6) | 4 (6.8) |
| Grade 3–5 | 0 | 0 | |
| Thrombocytopenia | Any grade | 27 (16.4) | 11 (18.6) |
| Grade 3–5 | 7 (4.2) | 5 (8.5) | |
| Leukopenia | Any grade | 39 (23.6) | 10 (16.9) |
| Grade 3–5 | 6 (3.6) | 6 (10.2) | |
| ALT/AST elevated | Any grade | 19 (11.5) | 8 (13.6) |
| Grade 3–5 | 0 | 0 | |
| Nasal bleeding | Any grade | 1 (0.6) | 1 (1.7) |
| Grade 3–5 | 0 | 0 | |
| Nausea | Any grade | 6 (3.6) | 1 (1.7) |
| Grade 3–5 | 1 (0.6) | 2 (3.4) | |
| Joint pain | Any grade | 1 (0.6) | 1 (1.7) |
| Grade 3–5 | 1 (0.6) | 0 | |
| Rash | Any grade | 6 (3.6) | 0 |
| Grade 3–5 | 0 | 0 | |
| Diarrhea | Any grade | 4 (2.4) | 1 (1.7) |
| Grade 3–5 | 0 | 0 | |
| Hair loss | Any grade | 2 (1.2) | 0 |
| Grade 3–5 | 0 | 0 | |
| Poor appetite | Any grade | 4 (2.4) | 1 (1.7) |
| Grade 3–5 | 0 | 0 | |
| Fatigue | Any grade | 5 (3.0) | 1 (1.7) |
| Grade 3–5 | 2 (1.2) | 0 | |
| Bilirubin increased | Any grade | 4 (2.4) | 0 |
| Grade 3–5 | 0 | 0 | |
| Creatinine increased | Any grade | 10 (6.1) | 4 (6.8) |
| Grade 3–5 | 0 | 0 | |
| Proteinuria | Any grade | 1 (0.6) | 0 |
| Grade 3–5 | 0 | 0 | |
| Fever | Any grade | 3 (1.8) | 3 (5.1) |
| Grade 3–5 | 2 (1.2) | 2 (3.4) | |
| Paresthesia | Any grade | 1 (0.6) | 1 (1.7) |
| Grade 3–5 | 0 | 0 | |
| Hyperuricemia | Any grade | 0 | 1 (1.7) |
| Grade 3–5 | 0 | 1 (1.7) |
Data are presented as n (%). ICI, immune checkpoint inhibitor; ALT, alanine aminotransferase; AST, aspartate transaminase.
Table 6
| Events | Grade | Resistant group (N=51) | Responder group (N=114) |
|---|---|---|---|
| Hypothyroidism | Any grade | 3 (5.9) | 9 (7.9) |
| Grade 3–5 | 0 | 0 | |
| Neutropenia | Any grade | 9 (17.6) | 20 (17.5) |
| Grade 3–5 | 3 (5.9) | 7 (6.1) | |
| Pneumonia | Any grade | 4 (7.8) | 3 (2.6) |
| Grade 3–5 | 1 (2.0) | 2 (1.8) | |
| Anemia | Any grade | 13 (25.5) | 22 (19.3) |
| Grade 3–5 | 4 (7.8) | 3 (2.6) | |
| Arrhythmia | Any grade | 3 (5.9) | 4 (3.5) |
| Grade 3–5 | 0 | 0 | |
| Thrombocytopenia | Any grade | 13 (25.5) | 14 (12.3) |
| Grade 3–5 | 5 (9.8) | 2 (1.8) | |
| Leukopenia | Any grade | 14 (27.5) | 24 (21.1) |
| Grade 3–5 | 3 (5.9) | 3 (2.6) | |
| ALT/AST elevated | Any grade | 8 (15.7) | 12 (10.5) |
| Grade 3–5 | 0 | 0 | |
| Nasal bleeding | Any grade | 1 (2.0) | 0 |
| Grade 3–5 | 0 | 0 | |
| Nausea | Any grade | 3 (5.9) | 3 (2.6) |
| Grade 3–5 | 0 | 1 (0.9) | |
| Rash | Any grade | 1 (2.0) | 4 (3.5) |
| Grade 3–5 | 0 | 0 | |
| Diarrhea | Any grade | 0 | 2 (1.8) |
| Grade 3–5 | 0 | 0 | |
| Hair loss | Any grade | 1 (2.0) | 1 (0.9) |
| Grade 3–5 | 0 | 0 | |
| Poor appetite | Any grade | 1 (2.0) | 3 (2.6) |
| Grade 3–5 | 0 | 0 | |
| Fatigue | Any grade | 5 (9.8) | 4 (3.5) |
| Grade 3–5 | 2 (3.9) | 2 (1.8) | |
| Creatinine increased | Any grade | 0 | 10 (8.8) |
| Grade 3–5 | 0 | 0 | |
| Fever | Any grade | 0 | 3 (2.6) |
| Grade 3–5 | 0 | 2 (1.8) | |
| Paresthesia | Any grade | 0 | 1 (0.9) |
| Grade 3–5 | 0 | 0 |
Data are presented as n (%). ALT, alanine aminotransferase; AST, aspartate transaminase.
Discussion
In this retrospective study, we explored the efficacy and safety of different second-line treatment regimens for locally advanced and advanced NSCLC. Our results suggested that ICI rechallenge therapy after disease progression did not provide clinical benefit but displaying a manageable safety profile. Response to prior immunotherapy correlated with the efficacy of second-line ICI rechallenge therapy.
Previous studies have demonstrated that there is an important relationship between the tumor immune microenvironment and tumor angiogenesis, and inhibition of neovascularization can improve the tumor immune microenvironment, which promotes the infiltration of T-lymphocytes into the tumor microenvironment and improves the efficacy of immunotherapy (12,13). The results of the IMpower150 study demonstrated that the efficacy of a combination of atezolizumab, bevacizumab, carboplatin, and paclitaxel may lead to significant improvements in PFS and OS in patients with metastatic NSCLC (15). Lung-MAP S1800A is a randomized phase II clinical study of ramucirumab in combination with pembrolizumab versus standard of care in advanced NSCLC previously treated with immunotherapy. The results of the study showed a mPFS of 4.5 months and an ORR of 22% in the pembrolizumab combined with ramucirumab treatment group (18). The study suggested that pembrolizumab in combination with ramucirumab improved survival in patients with advanced NSCLC who had received prior immunotherapy and chemotherapy. Results from the SCORPION study, a multicenter phase II study of the combination of docetaxel and ramucirumab in NSCLC patients beyond disease progression on first-line chemotherapy plus ICIs, showed an ORR of 34.4%, which was higher than our study (ORR =21.1%) (16). This discrepancy may be related to the fact that in the real-world setting, patients whose health has been declining because of the disease progression on first-line treatment, are generally less tolerant of second-line treatment. The results showed that docetaxel in combination with ramucirumab demonstrated encouraging antitumor activity and manageable safety profile in patients who failed first-line chemotherapy plus ICIs. Although the data analysis among different second-line treatment options was not statistically significant in our study, the ICIs + A group achieved the longest PFS2 of 6.64 months (95% CI: 5.27–8.00), followed by C + A group of 6.41 months (95% CI: 3.91–8.90). Therefore, the efficacy of the antiangiogenic drugs in combination with ICIs or chemotherapy may still worth exploring in second-line settings.
Meanwhile, our findings showed that the PFS2 obtained in the ICI monotherapy group was 3.68 months (95% CI: 1.99–5.37). A pooled analysis based on the five KEYNOTE series of studies showed that patients with PD showed a survival benefit in second-line pembrolizumab monotherapy after completing 35 cycles of pembrolizumab with or without chemotherapy (19). The mPFS in the ICI monotherapy of this pooled analysis was longer than in our study (10.3 vs. 3.68 months). We attribute this difference to the high proportion of patients with PD-L1 tumor proportion score (TPS) ≥50% in the ICI monotherapy group of this pooled analysis, of which 47/58 (81%) of patients had PD-L1 TPS of 50% or greater. However, we have not determined the efficacy difference at different PD-L1 expression levels because of the limited number of patients with known PD-L1 expression levels. Therefore, further studies are still needed to confirm whether patients with high PD-L1 expression can benefit from second-line ICI monotherapy. In our study, the chemotherapy alone group has achieved the worst PFS2 (mPFS2 =3.22 months, 95% CI: 2.28–4.16). According to European Society for Medical Oncology (ESMO) Clinical Practice Guidelines, the use of platinum-based chemotherapy after disease progression is preferred. A multicentric international study analyzed the efficacy of second-line chemotherapy regimens after disease progression, mPFS in the chemotherapy alone group was 2.9 months (95% CI: 2.4–3.3), which was very similar to our results (3.22 months) (20). Our study confirmed that the use of chemotherapy alone in the second line did not provide significant benefit to patients.
In the analysis of Cohort C of the VARGADO study (NCT02392455), nintedanib in combination with docetaxel after the first-line treatment of ICIs combination chemotherapy showed that ECOG PS >1 was associated with lower ORR, DCR and shorter PFS and OS. In our study, better physical conditions were associated with higher ORR, DCR, and relatively longer PFS2. A systematic review of the efficacy and safety of ICI rechallenge in NSCLC also suggested that patients with good ECOG PS had better outcomes (21). Radiotherapy can enhance tumor antigen presentation and induce anti-tumor T cell responses to maximize control tumor, and radiotherapy can heighten the effect of immunotherapy (22-24). Several studies have demonstrated that the combination of radiotherapy and immunotherapy prolongs PFS and OS in NSCLC patients (25,26). Given the sensitizing mechanism of radiotherapy to immunotherapy, it is also widely accepted by clinicians that all patients can benefit from second-line combination of radiotherapy after disease progression on first-line immunotherapy. A phase II prospective trial enrolled NSCLC patients treated with first-line pembrolizumab, and 21 patients received stereotactic body radiotherapy (SBRT) after disease progression. The final results showed that the addition of SBRT after progression on a PD-1 inhibitor prolonged PFS with favorable efficacy (27). Our study showed that patients who received radiotherapy during second-line treatment had significantly higher ORR and DCR and longer PFS2 than those without radiotherapy. However, multivariate analysis showed that the combination of radiotherapy during the second-line treatment was not a major factor influencing PFS2 of all patients. Similar to our study, results of a phase II study exploring treatment strategies for different oligoprogression patterns after failure of immunotherapy in metastatic NSCLC showed that only repeat oligoprogression group (diagnosis of oligoprogression with a history of oligometastatic disease) had a survival benefit from the addition of local therapy, and the benefit was not apparent in other metastatic NSCLC situations (28). The results of our study likewise confirmed this idea that local therapy during second-line treatment may benefit only a small percentage of patients. More studies of such patients are needed in the future for further clarification.
In addition, our analysis showed no significant differences in mPFS2 and ORR between the ICI rechallenge group and non-rechallenge group. Patients with locally advanced or advanced NSCLC who continued immunotherapy beyond PD after the first-line treatment showed a negative clinical benefit. Patients who received ICI rechallenge had a lower ORR compared with those who did not receive second-line immunotherapy (10.3% vs. 15.3%), although the difference was not statistically significant. The median PFS2 was 5.33 months (95% CI: 4.06–6.58) for patients who received ICI rechallenge and 4.40 months (95% CI: 2.67–6.14) for those who did not receive immunotherapy after failure of first-line treatment (HR =1.062, 95% CI: 0.77–1.47; P=0.715). Results from a retrospective study of the safety and efficacy of continuing ICIs in patients with disease progression after first-line ICIs plus chemotherapy, which included 59 patients, also suggested that continuing ICI rechallenge in NSCLC patients after initial disease progression did not improve clinical outcomes (29). Compared with this retrospective analysis, our study expands the sample size and reduces errors due to insufficient quantities. However, the results of the retrospective study showed that antiangiogenic drugs in combination with ICIs had the worst PFS2 (mPFS2 =1.41 months), which was completely different from our study that the ICIs + A group obtained the longest PFS2 (mPFS2 =6.64 months). We believe that this difference may be due to differences in baseline characteristics. Another retrospective study of ICI rechallenge in 40 NSCLC patients showed longer mPFS and ORR than ours (6.8 vs. 5.33 months; 22.5% vs. 10.3%) (30). There are several possible reasons for this discrepancy. Our study enrolled patients with unresectable stage III or IV NSCLC, while this study included early-stage NSCLC patients. In addition, our study enrolled patients without driver mutations and were directly rechallenged with ICI beyond PD after the first-line immunotherapy treatment. While this study included 17 patients who carried mutated genes and seven patients received chemotherapy or targeted therapy prior to ICI rechallenge treatment. Although the study suggested that ICI rechallenge may be an option for NSCLC after progress to immunotherapy, the results of all prognostic factors were nonsignificant. Further prospective studies with larger sample size are needed.
Among the 165 patients who received ICI rechallenge in the second-line treatment, the median PFS2 was 3.68 months (95% CI: 2.46–4.90) in the resistant group and 5.91 months (95% CI: 5.29–6.54) in the responder group (P=0.014). Multivariate analysis of the ICI rechallenge group (n=165) also showed that response to initial ICIs treatment was an independent factor associated with PFS2 (P=0.015). The results suggested that the efficacy of ICI rechallenge correlated with response to first-line immunotherapy. A Japanese study of PD-L1 rechallenge in 17 patients with advanced NSCLC showed that a good response of initial ICIs treatment may be one of the clinical features that predicts the efficacy of subsequent ICI rechallenge (31). Similarly, the results of a systematic review including 2,100 patients from 17 studies demonstrated the length of PFS1 was an independent prognostic factors of PFS2 (P=0.006) (32). Our study provides valuable recommendations for clinical practice that NSCLC patients who respond better to first-line immunotherapy can still benefit from ICI rechallenge.
Similar incidences of AEs at any grade were observed in the different second-line treatment groups (P=0.334), and most of them were classified as grade 1 or 2 AEs. Similarly, no statistically significant differences in the incidence of grade 3 and 4 AEs were observed (P=0.162). In the C + A group, two of 38 (5.3%) patients experienced grade 4 TRAEs. The two cases were myelosuppression and hyperuricosuria, respectively. In the ICIs + A group, three of 77 (3.9%) patients experienced grade 4 TRAEs as myelosuppression. In the ICIs + C + A group, four of 51 (7.8%) patients experienced grade 4 myelosuppression and one of them died within three months after the initiation of second-line treatment because of the severe AEs. Similar incidences of TRAEs at any grade were observed in ICI rechallenge group and non-rechallenge group (63.0% vs. 71.2%, P=0.259), and no statistically significant differences in the incidence of grade 3 and 4 AEs were observed between the two groups (15.2% vs. 22.0%, P=0.227). The ICI rechallenge group showed no new safety signals compared with patients who discontinued ICIs treatment. Similarly, second-line ICI rechallenge therapy showed acceptable toxicity profile in patients with a good response to first-line immunotherapy.
PD-L1 expression levels, tumor mutational burden (TMB), and tumor-infiltrating lymphocytes (TILs) are regarded as some of the biomarkers predicting the efficacy of ICIs treatment. PD-L1 is a surface molecule which is expressed on different types of cells. Many studies have confirmed that PD-L1 expression was a predictive biomarker for the efficacy of ICIs (33,34). In 2018, Rizvi et al. (35) observed a correlation between high TMB and clinical outcomes for patients with NSCLC on immunotherapy treatment, which confirmed that high TMB tended to predict the efficacy of immunotherapy. TILs play a key role in the immunogenic reaction against tumors, and several studies supported TILs as a prognostic and predictive biomarker (36,37). However, the identification and testing of robust and reliable predictive biomarkers are not sufficiently precise. Due to insufficient information on such biomarkers in patients enrolled in our study, we did not explore meaningful biomarkers in patients who responded well to initial ICIs treatment.
In spite of the fact that our study provides several significant references for the selection of second-line treatment options beyond disease progression, there are several limitations. First, stratified analysis of PD-L1 expression was not available because some patients were not tested for PD-L1 expression, which may have overlooked the impact on ICI monotherapy efficacy. Second, this was a clinical retrospective study, which may lead to selection bias. Further studies should examine efficacy and safety of different second-line treatment regimens in large randomized prospective cohorts, and determine which patient groups can easily benefit from ICI rechallenge therapy.
Conclusions
Our study suggested that ICI rechallenge therapy beyond disease progression did not improve clinical outcomes in patients with NSCLC, but no new safety signals emerged. However, some patients can benefit from immunotherapy rechallenge. In our study, patients with favorable response to initial ICIs treatment still showed significant efficacy and acceptable toxicity profile of subsequent ICI rechallenge therapy. Considering that the optimal choice for second-line treatment after initial treatment with ICIs remains inconclusive, our study may provide important references for clinical decision-making.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1767/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1767/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1767/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1767/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Academic Ethics Committee of Jiangsu Cancer Hospital [No. (2023)082] and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc 2019;94:1623-40. [Crossref] [PubMed]
- Huang Z, Su W, Lu T, et al. First-Line Immune-Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Current Landscape and Future Progress. Front Pharmacol 2020;11:578091.
- Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med 2020;383:1328-39. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50. J Clin Oncol 2021;39:2339-49. [Crossref] [PubMed]
- Schoenfeld AJ, Hellmann MD. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020;37:443-55. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50.
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Wu YL, Lu S, Cheng Y, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol 2019;14:867-75. [Crossref] [PubMed]
- Bernard-Tessier A, Baldini C, Martin P, et al. Outcomes of long-term responders to anti-programmed death 1 and anti-programmed death ligand 1 when being rechallenged with the same anti-programmed death 1 and anti-programmed death ligand 1 at progression. Eur J Cancer 2018;101:160-4. [Crossref] [PubMed]
- Lebbé C, Weber JS, Maio M, et al. Survival follow-up and ipilimumab retreatment of patients with advanced melanoma who received ipilimumab in prior phase II studies. Ann Oncol 2014;25:2277-84. [Crossref] [PubMed]
- Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018;15:325-40. [Crossref] [PubMed]
- Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A 2012;109:17561-6.
- Choi SH, Yoo SS, Lee SY, et al. Anti-angiogenesis revisited: reshaping the treatment landscape of advanced non-small cell lung cancer. Arch Pharm Res 2022;45:263-79. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Matsuzawa R, Morise M, Ito K, et al. 46P Multi-center, phase II study of docetaxel (DTX) plus ramucirumab (RAM) following platinum-based chemotherapy plus ICIs in patients with NSCLC: SCORPION study. J Thorac Oncol 2023;18:S35-88.
- Grohé C, Wehler T, Dechow T, et al. Nintedanib plus docetaxel after progression on first-line immunochemotherapy in patients with lung adenocarcinoma: Cohort C of the non-interventional study, VARGADO. Transl Lung Cancer Res 2022;11:2010-21. [Crossref] [PubMed]
- Reckamp KL, Redman MW, Dragnev KH, et al. Phase II Randomized Study of Ramucirumab and Pembrolizumab Versus Standard of Care in Advanced Non-Small-Cell Lung Cancer Previously Treated With Immunotherapy-Lung-MAP S1800A. J Clin Oncol 2022;40:2295-306. [Crossref] [PubMed]
- Rodriguez-Abreu D, Wu YL, Boyer M, et al. OA15.06 Pooled Analysis of Outcomes with Second-Course Pembrolizumab Across 5 Phase 3 Studies of Non-Small-Cell Lung Cancer. J Thorac Oncol 2022;17:S42-3.
- Auclin E, Benitez-Montanez J, Tagliamento M, et al. Second-line treatment outcomes after progression from first-line chemotherapy plus immunotherapy in patients with advanced non-small cell lung cancer. Lung Cancer 2023;178:116-22.
- Plazy C, Hannani D, Gobbini E. Immune Checkpoint Inhibitor Rechallenge and Resumption: a Systematic Review. Curr Oncol Rep 2022;24:1095-106. [Crossref] [PubMed]
- Kono K, Mimura K, Kiessling R. Immunogenic tumor cell death induced by chemoradiotherapy: molecular mechanisms and a clinical translation. Cell Death Dis 2013;4:e688. [Crossref] [PubMed]
- Kalbasi A, June CH, Haas N, et al. Radiation and immunotherapy: a synergistic combination. J Clin Invest 2013;123:2756-63.
- Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259-71. [Crossref] [PubMed]
- Jabbour SK, Lee KH, Frost N, et al. Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients With Unresectable, Locally Advanced, Stage III Non-Small Cell Lung Cancer: The Phase 2 KEYNOTE-799 Nonrandomized Trial. JAMA Oncol 2021; Epub ahead of print. [Crossref]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. [Crossref] [PubMed]
- Campbell AM, Cai WL, Burkhardt D, et al. Final Results of a Phase II Prospective Trial Evaluating the Combination of Stereotactic Body Radiotherapy (SBRT) with Concurrent Pembrolizumab in Patients with Metastatic Non-Small Cell Lung Cancer (NSCLC). Int J Radiat Oncol Biol Phys 2019;105:S36-S37.
- Xuzhang W, Huang H, Yu Y, et al. Treatment strategies based on different oligoprogressive patterns after immunotherapy failure in metastatic NSCLC. Ther Adv Med Oncol 2023;15:17588359231156387. [Crossref] [PubMed]
- Wang Y, Fu S, Zhang X, et al. Continuation of anti-PD-1 therapy plus physician-choice treatment beyond first progression is not associated with clinical benefit in patients with advanced non-small cell lung cancer. Front Immunol 2023;14:1151385. [Crossref] [PubMed]
- Xu Z, Hao X, Yang K, et al. Immune checkpoint inhibitor rechallenge in advanced or metastatic non-small cell lung cancer: a retrospective cohort study. J Cancer Res Clin Oncol 2022;148:3081-9. [Crossref] [PubMed]
- Kitagawa S, Hakozaki T, Kitadai R, et al. Switching administration of anti-PD-1 and anti-PD-L1 antibodies as immune checkpoint inhibitor rechallenge in individuals with advanced non-small cell lung cancer: Case series and literature review. Thorac Cancer 2020;11:1927-33. [Crossref] [PubMed]
- Feng Y, Tao Y, Chen H, et al. Efficacy and safety of immune checkpoint inhibitor rechallenge in non-small cell lung cancer: A systematic review and meta-analysis. Thorac Cancer 2023;14:2536-47. [Crossref] [PubMed]
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Rizvi H, Sanchez-Vega F, La K, et al. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients With Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J Clin Oncol 2018;36:633-41. Erratum: J Clin Oncol 2018;36:1645. [Crossref] [PubMed]
- Liu C, Zheng S, Wang Z, et al. KRAS-G12D mutation drives immune suppression and the primary resistance of anti-PD-1/PD-L1 immunotherapy in non-small cell lung cancer. Cancer Commun (Lond) 2022;42:828-47. [Crossref] [PubMed]
- Gataa I, Mezquita L, Rossoni C, et al. Tumour-infiltrating lymphocyte density is associated with favourable outcome in patients with advanced non-small cell lung cancer treated with immunotherapy. Eur J Cancer 2021;145:221-9. [Crossref] [PubMed]


