Clinical impact of postoperative radiotherapy in pIII-N2 non-small cell lung cancer after complete resection followed by adjuvant chemotherapy: a systematic review and meta-analysis
Highlight box
Key findings
• Additional postoperative radiotherapy (PORT) was significantly associated with a reduced local recurrence rate while this does not result in improved survival outcomes in patients with completely resected pIII-N2 non-small cell lung cancer (NSCLC).
What is known and what is new?
• PORT may reduce the risk of local recurrence, but may cause damage to the lungs, heart, and other major organs. Owing to these factors, previous studies have not yielded consistent results regarding the clinical impact of PORT in patients with completely resected pIII-N2 NSCLC.
• A meta-analysis including recently performed large-scale studies highlighted that additional PORT does not provide a survival benefit.
What is the implication, and what should change now?
• PORT would not be routinely recommended for patients with completely resected pIII-N2 NSCLC, and further prospective studies are needed to identify subgroups of patients for whom PORT would be most beneficial.
Introduction
The prognosis for patients with pathological stage III-N2 (pIII-N2) non-small cell lung cancer (NSCLC) remains poor, with 5-year survival rates ranging from 19.2% to 30% (1,2). Although this is mainly due to the high risk of distant metastasis, locoregional recurrence rates remain high even after complete resection. Multimodal therapy is thought to offer the best chance for improving the prognosis of pIII-N2 NSCLC (3,4).
Large clinical trials have demonstrated that adjuvant chemotherapy offers clinical benefits in terms of overall survival (OS) and disease-free survival (DFS) among patients with completely resected pIII-N2 NSCLC. As a result, adjuvant chemotherapy has become the standard treatment for these patients (5-7). However, even with adjuvant chemotherapy in completely resected pIII-N2, the incidence of local recurrence remains high, ranging from 20% to 40% (6,8). Theoretically, postoperative radiotherapy (PORT) could reduce the risk of local recurrence and further improve survival outcomes (9,10). On the other hand, PORT may cause damage to the lungs, heart, and other major organs, potentially worsening the prognosis. Owing to these factors, previous studies have not yielded consistent results regarding the clinical impact of PORT in patients with completely resected pIII-N2 NSCLC (9-16).
Recently, two large-scale phase 3 randomized clinical trials (RCTs) were published, showing that PORT was not associated with improved DFS or OS in patients who underwent adjuvant chemotherapy following complete resection of pIIIA-N2 NSCLC (17,18). Including these trials, this meta-analysis of currently available findings from RCTs was performed to evaluate the clinical impact of PORT in these patients. We present this article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1742/rc) (19,20).
Methods
Data sources and searches
A systematic literature search was performed using PubMed, Embase, and the Cochrane Central Register of Controlled Trials, to identify studies published up to November 16, 2022. The search process adhered to the PRISMA guidelines. The main search terms used in various combinations included “non-small cell lung cancer”, “NSCLC”, “postoperative radiotherapy”, “PORT”, “stage III”, “N2”, and “randomized controlled trial”. Further details of the search strategy are shown in Tables S1-S3. The reference lists of retrieved studies were manually reviewed for additional relevant studies missing from the electronic search. Two researchers (I.H.K. and J.K.Y.) independently conducted the literature retrieval. Ethical approval was not required for this study, as all data were derived from public databases.
Study selection
Studies were included if they met the following criteria: (I) types of studies: RCT; (II) types of participants: completely resected pIII-N2 NSCLC; (III) types of interventions: compared surgical resection with or without PORT according to clinicopathological features; and (IV) outcomes: reported survival (OS and/or DFS) data. If the same study population was covered by multiple articles, the most recent article with complete survival data was chosen. Studies were excluded for any of the following reasons: (I) letters, editorials, case reports, and reviews; and (II) survival data could not be extracted from the literature.
Data extraction and quality assessment
Data extraction and the methodological quality of RCTs were independently performed by two investigators independently (I.H.K. and J.K.Y.). The methodological quality of RCTs was assessed using a Cochrane risk of bias tool (21), in accordance with the following seven domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; and selective reporting; other bias. In case of any discrepancies, a consensus was reached through discussion.
Statistical analyses
The hazard ratio (HR) and its 95% confidence intervals (CIs) were used to measure survival outcomes such as DFS and OS. An HR of <1 for DFS and OS was deemed preferable for PORT. For binary outcomes such as local recurrence, distant metastasis, and treatment-related adverse events (AEs), the odds ratio (OR) and their 95% CIs were applied to measure outcomes and safety. An OR of <1 for local recurrence and distant metastasis was considered preferable for PORT. Statistical test for heterogeneity was performed using the chi-square (χ2) and I-square (I2) tests with the significance set at P<0.10 and/or I2>50%. If there was statistical heterogeneity (I2≥50% and/or P<0.10) among studies, a random-effects model was used for OR and HR analysis; otherwise, a fixed-effects model was applied. Forest plots were drawn to show the estimated ORs, HRs, and their 95% CIs, representing the theoretical gain in absolute percentage based on endpoints. The funnel plot, Begg’s test, and Egger’s linear regression test were used to investigate any potential publication bias (22,23). All statistical calculations were performed with R (version 4.1.0; The R Foundation for Statistical Computing, Vienna, Austria). P values were two-tailed and a P value of <0.05 was considered statistically significant.
Results
Literature search and study characteristics
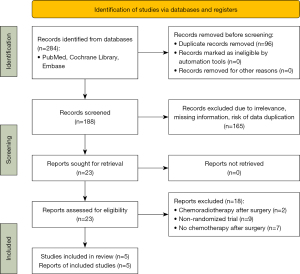
A total of 284 articles were retrieved. After excluding 96 duplicate articles based on title, year, and author information, 183 additional articles were removed following the abstract and full-text screening, leaving five studies for inclusion (17,18,24-26) (Figure 1). These studies encompassed 1,138 patients with stage III-N2 NSCLC; 572 patients received PORT, and 566 patients did not. All included studies were of high quality, as shown in Figure 2. Key baseline characteristics of the patients are comprehensively detailed in Table 1.
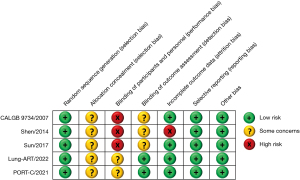
Table 1
| Study [year] | Time duration | Country of origin | Study design | No. of patients | Histology | No. of patients (PORT) | No. of patients (non-PORT) | RT technique | RT dose | RT timing | Type of surgery | Chemotherapy regimen | Outcomes (PORT vs. non-PORT) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CALGB 9734 [2007] | 1998–2000 | America | Phase III, single center, RCT |
37 | NR | 19 | 18 | NR | 50 Gy | Continuous (2–4 weeks after CTx) |
Lob/Bil/Pne | 4 cycles of paclitaxel (200 mg/m2)/carboplatin (AUC <6) | 1-year OS: 74% vs. 72%; median DFS: 33.7 vs. 16.8 months |
| Shen [2014] | 2004–2009 | China | Phase III, multi-center, RCT |
135 | SCC: 59 (43.7%); NSCC: 76 (56.3%) | 66 | 69 | 3D | 50 Gy | Concurrent | Lob/Bil/Pne | 4 cycles of paclitaxel (175 mg/m2)/cisplatin (60 mg/m2) | 5-year OS: 37.9% vs. 27.5%; median OS: 40 vs. 28 months; 5-year DFS: 30.3% vs. 18.8%; median DFS: 28 vs. 18 months |
| Sun [2017] | 2009–2014 | Korea | Phase II, single center, RCT |
101 | SCC: 20 (19.8%); NSCC: 81 (80.2%) | 51 | 50 | 3D | 50 Gy | Concurrent | Seg/Lob/Bil/Pne | PORT: 5 cycles of weekly paclitaxel (50 mg/m2)/cisplatin (25 mg/m2) +2 cycles of paclitaxel (175 mg/m2)/cisplatin (60 mg/m2); non-PORT: 4 cycles of paclitaxel (175 mg/m2)/carboplatin (AUC =5.5) | Median OS: 74.3 vs. 83.5 months; median DFS: 24.7 vs. 21.9 months |
| Lung-ART [2022] | 2007–2018 | France, UK, Germany, Switzerland, Belgium | Phase III, multi-center, RCT |
501 | SCC: 108 (21.6%); NSCC: 393 (78.4%) | 252 | 249 | 3D (89%), IMRT (11%) |
54 Gy | Continuous (2–6 weeks after CTx) |
Sub/Lob/Pne | Platinum-based doublets (neoadjuvant or adjuvant) | 3-year OS: 66.5% vs. 68.5%; 3-year DFS: 47.1% vs. 43.8%; median DFS: 30.5 vs. 22.8 months |
| PORT-C [2021] | 2009–2017 | China | Phase III, multi-center, RCT |
364 | SCC: 59 (16.2%); NSCC: 305 (83.8%) | 184 | 180 | 3D (11%) or IMRT (89%) | 50 Gy | Continuous (<6 weeks after CTx) |
Lob/Bil | 4 cycles of platinum-based doublet regimen | 3-year OS: 78.3% vs. 82.8%; 3-year DFS: 40.5% vs. 32.7%; median DFS: 22.1 vs. 18.6 months |
PORT, postoperative radiotherapy; RT, radiotherapy; RCT, randomized controlled trial; NR, not reported; CTx, chemotherapy; Lob, lobectomy; Bil, bilobectomy; Pne, pneumonectomy; OS, overall survival; DFS, disease-free survival; SCC, squamous cell carcinoma; NSCC, non-squamous cell carcinoma; 3D, three-dimensional conformed radiotherapy; IMRT, intensity-modulated radiotherapy; Seg, segmentectomy; Sub, sublobar resection; AUC, area under the curve.
Effect of PORT on DFS and OS
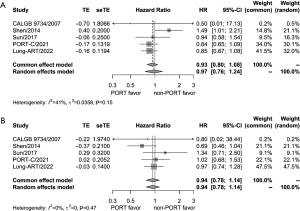
Data on DFS and OS were available from all the five trials. Based on the results of the heterogeneity test (P=0.15, I2=41% for DFS; P=0.47, I2=0% for OS), both DFS and OS were analyzed by the fixed-effects model. For DFS, there was no significant difference between the PORT group and the observation group (HR =0.93, 95% CI: 0.80–1.08) (Figure 3A). Similarly, OS was not significantly different between the two groups (HR =0.94, 95% CI: 0.78–1.14) (Figure 3B).
Effect of PORT on other outcomes
Regarding local and distant recurrence, no significant heterogeneity was found among the five trials (P=0.44, I2=0% for local recurrence; P=0.39, I2=3% for distant recurrence). A fixed-effects model was used for analysis. The rate of local recurrence was significantly lower in the PORT group compared to those of the observation group (OR =0.53, 95% CI: 0.40–0.70) (Figure 4A). Meanwhile, there was no significant difference of distant recurrence rate between the two groups (OR =0.94, 95% CI: 0.74–1.20) (Figure 4B).
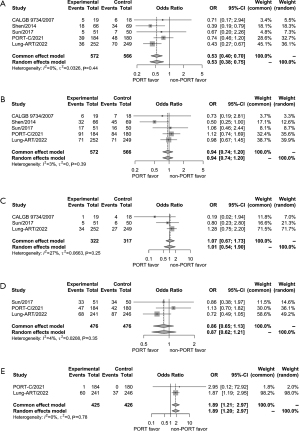
Information on brain metastasis was available from three trials (17,24,26). Based on the results of the heterogeneity test (P=0.25, I2=27%), a fixed-effects model was used for analysis. The rate of brain metastasis was not significantly different between the PORT group and the observation group (OR =1.07, 95% CI: 0.67–1.73) (Figure 4C). Data on cancer death were available from three trials (17,18,26). No significant heterogeneity was observed (P=0.35, I2=4%). Using the fixed-effects model, no difference was found in the rate of cancer deaths between the two groups (OR =0.86, 95% CI: 0.65–1.13) (Figure 4D). Regarding adverse outcomes of grade 3 or higher, only two trials (PORT-C and Lung-ART) reported treatment-related adverse outcomes (17,18). According to the fixed-effects model, the rate of treatment-related adverse outcomes of grade 3 or higher increased in the PORT group compared to those of the observation group (OR =1.89, 95% CI: 1.21–2.97) (Figure 4E).
Sensitivity analysis and publication bias
Sensitivity analysis involving the five trials was conducted by excluding one study at a time to evaluate result stability. The corresponding HR and OR values remained largely unchanged, indicating stable results (Figure S1). The funnel plot was used to assess the likelihood of publication bias; no evident asymmetry was observed, suggesting a low likelihood of such bias (Figure S2).
Discussion
Monotherapy with surgical resection for pIII-N2 NSCLC is considered to have limited therapeutic effects, even when complete resection is performed (3,27). In other words, multimodal therapy is required to achieve the best survival outcomes in this patient group. However, the optimal combination of multimodal therapy remains unclear owing to the clinical heterogeneity in patients with pIII-N2 NSCLC. Although adjuvant chemotherapy is widely accepted as the standard treatment, the potential survival benefits of PORT are still debated in patients with pIII-N2 NSCLC. In this study, we included 5 RCTs involving 1,138 patients to evaluate the prognostic impact of PORT in pIII-N2 NSCLC after complete resection followed by adjuvant chemotherapy. The results indicated a significant reduction in local recurrence rates associated with PORT, although both DFS and OS did not benefit. By including two recently published large-scale studies (PORT-C and Lung-ART), this study aimed to provide comprehensive, updated, and reliable information on the effectiveness of PORT in pIII-N2 NSCLC.
Over the past 30 years, the role of PORT in pIII-N2 NSCLC after complete resection has been consistently controversial. Data from RCTs performed in the 1980 to 1990s were incorporated in a meta-analysis published in 1998 that showed worse outcomes for pN0 and pN1 NSCLC (28). Furthermore, it suggested a tendency for PORT to become increasingly detrimental as the number of involved lymph nodes (LNs) decreased, with a statistically significant trend observed (P=0.016). This was thought to be related to outdated radiation techniques and subsequent heart and lung toxicity supported by accumulating evidence from several other studies (29). Nonetheless, the meta-analysis in 1998 did not show an adverse effect of PORT in patients with stage III-N2. It implies the possibility that there might be oncological benefits that could compensate for the adverse effect of PORT in stage III-N2 NSCLC. Since then, radiotherapy techniques have been developed that are more delicate for target mediastinal tissue and less invasive for normal tissues including lung and heart (17,18,26). A further meta-analysis reported that decreased local relapse and increased OS when using PORT in stage IIIA-N2 NSCLC are attributed to the development of these technical aspects (12). Despite these advancements, concerns about toxicity persist, making clinicians reluctant to routinely use PORT in patients with completely resected N2 NSCLC in the era of conformal radiotherapy.
A majority of patients included in our meta-analysis (n=1,101/1,138, 96.7%) received either 3-dimensional conformal radiotherapy or intensity-modulated radiotherapy, which is known to ensure sufficient irradiation doses to the target volume and reduce risk to normal organ (30). However, a reduction in local recurrence rate did not finally lead to an improvement in DFS or OS. Given the heterogeneity among patients with pIII-N2 NSCLC, it could be inferred that only some but not all patients could benefit from PORT. In the PORT-C trial, a preplanned exploratory analysis revealed that DFS in patients with positive LNs ≥4 was significantly better than those with 1 to 3 LNs (18). A recent study using the National Cancer Database also reported that PORT was associated with improved OS only in patients with positive LNs ≥3 (HR: 0.91; 95% CI: 0.86–0.97; P=0.002). (31). Regarding the LN station, several studies revealed that PORT in completely resected stage III-N2 NSCLC improved both DFS and OS in patients with multiple N2 station metastases compared with single N2 station metastases (16,32).
Meanwhile, molecular markers might play a critical role in identifying patients who would benefit most from PORT. In the Impower010 trial, a significant increase of DFS was observed when adjuvant atezolizumab was additionally used after adjuvant chemotherapy in patients with resected stage II–IIIA NSCLC compared to best supportive group, with pronounced benefit in the programmed cell death-ligand 1 (PD-L1) tumor cells ≥1% subgroup (33). In addition, the ADAURA trial showed a DFS benefit with osimertinib in patients with resectable tumors harboring epidermal growth factor receptor (EGFR) mutations (34). Considering the therapeutic efficacy of immunotherapy and targeted therapy, PORT may offer limited benefit on oncological outcomes in these patients. Therefore, suggesting PORT only for patients with resected stage II–IIIA NSCLC who are not eligible for immunotherapy and targeted therapy would be reasonable.
This meta-analysis has several notable limitations. First, it was not feasible to perform subgroup analysis according to age, sex, smoking status, histology, and the number of LNs owing to the limited individual patient data. Second, inconsistencies in treatment policies, such as chemotherapy cycles, chemotherapy regimens and radiation doses, across the included studies could introduce heterogeneity. Third, our study only included five RCTs published in English, making it susceptible to language and publication biases.
Conclusions
Compared with adjuvant chemotherapy alone, the addition of PORT could reduce the incidence of local recurrence in patients with pIII-N2 NSCLC after complete resection. However, this does not result in improved OS or DFS. Given the efficacy of immunotherapy and target therapy, further prospective RCTs are needed to identify the subgroups of patients with pIII-N2 NSCLC for whom PORT would be most beneficial.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1742/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1742/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1742/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kirmani BH, Rintoul RC, Win T, et al. Stage migration: results of lymph node dissection in the era of modern imaging and invasive staging for lung cancer. Eur J Cardiothorac Surg 2013;43:104-9; discussion 109-10. [Crossref] [PubMed]
- Okada M, Tsubota N, Yoshimura M, et al. Prognosis of completely resected pN2 non-small cell lung carcinomas: What is the significant node that affects survival? J Thorac Cardiovasc Surg 1999;118:270-5. [Crossref] [PubMed]
- Yun JK, Bok JS, Lee GD, et al. Long-term outcomes of upfront surgery in patients with resectable pathological N2 non-small-cell lung cancer. Eur J Cardiothorac Surg 2020;58:59-69. [Crossref] [PubMed]
- Eberhardt WE, De Ruysscher D, Weder W, et al. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol 2015;26:1573-88. [Crossref] [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. Erratum in: Lancet Oncol 2006;7:797.
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [Crossref] [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [Crossref] [PubMed]
- Le Péchoux C. Role of postoperative radiotherapy in resected non-small cell lung cancer: a reassessment based on new data. Oncologist 2011;16:672-81. [Crossref] [PubMed]
- Dai H, Hui Z, Ji W, et al. Postoperative radiotherapy for resected pathological stage IIIA-N2 non-small cell lung cancer: a retrospective study of 221 cases from a single institution. Oncologist 2011;16:641-50. [Crossref] [PubMed]
- Corso CD, Rutter CE, Wilson LD, et al. Re-evaluation of the role of postoperative radiotherapy and the impact of radiation dose for non-small-cell lung cancer using the National Cancer Database. J Thorac Oncol 2015;10:148-55. [Crossref] [PubMed]
- Robinson CG, Patel AP, Bradley JD, et al. Postoperative radiotherapy for pathologic N2 non-small-cell lung cancer treated with adjuvant chemotherapy: a review of the National Cancer Data Base. J Clin Oncol 2015;33:870-6. [Crossref] [PubMed]
- Billiet C, Decaluwé H, Peeters S, et al. Modern post-operative radiotherapy for stage III non-small cell lung cancer may improve local control and survival: a meta-analysis. Radiother Oncol 2014;110:3-8. [Crossref] [PubMed]
- Sakib N, Li N, Zhu X, et al. Effect of postoperative radiotherapy on outcome in resectable stage IIIA-N2 non-small-cell lung cancer: an updated meta-analysis. Nucl Med Commun 2018;39:51-9. [Crossref] [PubMed]
- Lei T, Li J, Zhong H, et al. Postoperative Radiotherapy for Patients With Resectable Stage III-N2 Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front Oncol 2021;11:680615. [Crossref] [PubMed]
- Zhang H, Zhang DX, Ju T, et al. The effect of postoperative radiotherapy on the survival of patients with resectable stage III-N2 non-small-cell lung cancer: a systematic review and meta-analysis. Neoplasma 2019;66:717-26. [Crossref] [PubMed]
- Yun JK, Lee GD, Choi S, et al. The addition of radiotherapy to adjuvant chemotherapy has a combinatorial effect in pN2 non-small cell lung cancer only with extranodal invasion or multiple N2 metastasis. Lung Cancer 2021;155:94-102. [Crossref] [PubMed]
- Le Pechoux C, Pourel N, Barlesi F, et al. Postoperative radiotherapy versus no postoperative radiotherapy in patients with completely resected non-small-cell lung cancer and proven mediastinal N2 involvement (Lung ART): an open-label, randomised, phase 3 trial. Lancet Oncol 2022;23:104-14. [Crossref] [PubMed]
- Hui Z, Men Y, Hu C, et al. Effect of Postoperative Radiotherapy for Patients With pIIIA-N2 Non-Small Cell Lung Cancer After Complete Resection and Adjuvant Chemotherapy: The Phase 3 PORT-C Randomized Clinical Trial. JAMA Oncol 2021;7:1178-85. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [Crossref] [PubMed]
- Phan K, Tian DH, Cao C, et al. Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg 2015;4:112-22. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101.
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Perry MC, Kohman LJ, Bonner JA, et al. A phase III study of surgical resection and paclitaxel/carboplatin chemotherapy with or without adjuvant radiation therapy for resected stage III non-small-cell lung cancer: Cancer and Leukemia Group B 9734. Clin Lung Cancer 2007;8:268-72. [Crossref] [PubMed]
- Shen WY, Ji J, Zuo YS, et al. Comparison of efficacy for postoperative chemotherapy and concurrent radiochemotherapy in patients with IIIA-pN2 non-small cell lung cancer: an early closed randomized controlled trial. Radiother Oncol 2014;110:120-5. [Crossref] [PubMed]
- Sun JM, Noh JM, Oh D, et al. Randomized Phase II Trial Comparing Chemoradiotherapy with Chemotherapy for Completely Resected Unsuspected N2-Positive Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1806-13. [Crossref] [PubMed]
- Casali C, Stefani A, Natali P, et al. Prognostic factors in surgically resected N2 non-small cell lung cancer: the importance of patterns of mediastinal lymph nodes metastases. Eur J Cardiothorac Surg 2005;28:33-8. [Crossref] [PubMed]
- Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. PORT Meta-analysis Trialists Group. Lancet 1998;352:257-63.
- Banfill K, Giuliani M, Aznar M, et al. Cardiac Toxicity of Thoracic Radiotherapy: Existing Evidence and Future Directions. J Thorac Oncol 2021;16:216-27. [Crossref] [PubMed]
- Chen XR, Dong JN, Zhang F, et al. Efficacy and safety of image-guidance radiotherapy by helical tomotherapy in patients with lung cancer. Medicine (Baltimore) 2018;97:e9243. [Crossref] [PubMed]
- Zarinshenas R, Ladbury C, McGee H, et al. Machine learning to refine prognostic and predictive nodal burden thresholds for post-operative radiotherapy in completely resected stage III-N2 non-small cell lung cancer. Radiother Oncol 2022;173:10-8. [Crossref] [PubMed]
- Liu T, Mu Y, Dang J, et al. The role of postoperative radiotherapy for completely resected pIIIA-N2 non-small cell lung cancer patients with different clinicopathological features: a systemic review and meta-analysis. J Cancer 2019;10:3941-9. [Crossref] [PubMed]
- Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344-57. [Crossref] [PubMed]
- Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. [Crossref] [PubMed]