Complications of ultrasound guided very small-bore chest drains for pleural effusions of different etiology
Highlight box
Key findings
• This consecutive cohort of 484 very small chest drains [6–10 French (F)] in 330 people found that the intervention was related to a low risk of complications in simple pleural effusions (PEs), but high rates of complications and repeat intervention in empyema.
What is known and what is new?
• Small-bore chest drains (≤14 F) have shown comparable efficacy to larger-bore drains, but knowledge has been limited on very small drains.
• This study provides evidence on the clinical utility of very small chest drains by underlying etiology of the PE.
What is the implication, and what should change now?
• At the time of diagnosis, the clinician should categorize PEs as likely non-malignant, where very small chest drains are effective, and empyema which mandates the use of larger-bore drains and early contact with thoracic surgeon not to delay definitive treatment.
Introduction
Pleural effusions (PEs) are common in a vast array of patients in clinical care (1). Depending on the etiology and amount of pleural fluid, patients might require a chest drain to alleviate symptoms, drain infective foci, or to establish a diagnosis (1-4).
Small-bore chest drains, often defined as ≤14 French (F) of size, have become the most commonly used drains in the last decades. A large British audit in 2010 reported that 83% of all inserted chest drains were small-bore (5).
Historically, large-bore chest drains (16–40 F) were used to drain PE (6). Large-bore drains are inserted using blunt dissection technique and most often without image guidance. The more common small-bore chest drains are usually inserted with either Seldinger technique (over a guidewire introduced by a needle) or using a one-step technique (with support of a central metal trocar or hollow needle) (7-9). Image guidance for chest drain insertion is strongly recommended in guidelines and modality of choice varies between computer tomography, fluoroscopy, and, most commonly, ultrasound (4,10-15).
The main concern with using smaller drains, compared to larger ones, for complicated PE (e.g., empyema and malignant PE) is a potentially higher risk of drain blockage. Studies on the efficacy of small-bore drains on empyema show varying results with success rates reported between 40–92.5% and even though guidelines consider small-bore chest drains sufficient in early stages no consensus exists regarding ideal drain size (4,10,11,15-18). Studies regarding malignant PE and pleurodesis using small-bore drains show similar success rates to large-bore drains which has been reflected in guidelines and has facilitated an increased use of small-bore drains in clinical practice (8,15,19). Furthermore, multiple studies report positive results for small-bore drains in uncomplicated simple PE (e.g., secondary to heart failure) with a success rate similar to large-bore drains (7,20-22). In addition, the insertion and utilization of smaller drains results in less patient discomfort compared to larger ones (8,23,24). These findings have led to an increased use of very small drains (<10 F). Insertion of very small chest drains using one-step technique is often used at our Radiology Department as it is easy to learn, faster (compared to insertion using Seldinger technique), and considered safe as the use of ultrasound guidance allow imaging of the trocar tip during insertion.
However, data on complication rates and efficacy of these very small drains are limited, with most studies also including drains ranging 10–14 F (13,16,19), and this knowledge is important, especially given the potentially increased risk of complications of very small drains in malignant PE and empyema.
The primary aim of this study was to evaluate the rate of repeat intervention (need of surgery or new drain) and complications (blockage, dislodgement, misplacement or infection) of ultrasound guided very small-bore chest drains (sized 6–10 F), by etiology of the PE. Secondary aims were to evaluate rates of repeat intervention and complications by seniority of the radiologist performing the procedure and by drain size. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1457/rc).
Methods
Study design
This study was conducted as a retrospective study of all consecutive adult patients who received a chest drain (6–10 F) for PE at the Department of Radiology at Blekinge Hospital (situated in Karlskrona and Karlshamn), Sweden, between 1 January, 2018 and 31 December, 2020. The Department of Radiology at the Blekinge Hospital covers the whole region of approximately 159,000 residents (25).
We included all patients who received a chest drain regardless of referring clinic or suspected etiology of the PE. Patients with a pneumothorax or large hemothorax were not included since they routinely acquire large bore chest drains in our hospitals and are not referred to the Department of Radiology.
Procedure
Routine patient workup and eligibility criteria before drain insertion include a prothrombin international normalized ratio (PT/INR) <1.5, platelet count >50×109/L, blood count >80 g/L, and an activated partial thromboplastin time (APTT) <60 s. In patients in dire need of drainage, values outside these references might have been accepted at the discretion of the responsible clinician, and the rate of this was not recorded.
At the Department of Radiology only very small chest drains (6–10 F) were used during the study period. Chest drains were inserted using real time ultrasound guidance (at the time Logic 9, GE ultrasound) with the patient sitting upright and slightly hunchbacked. If the patient was unable to sit up, the procedure was carried out with the patient recumbent. The preferred insertion site was caudally in the posterior axillary line. The insertion site was prepped and draped in a sterile manner. A needle guide was used with the ultrasound when administering local anesthetics (1% Carbocaine, usually 5–10 mL) and inserting the drain using one-step technique [SKATER™ Single Step Drainage (nephrostomy), Plano, TX, USA, set locking—Argon Medical Devices, Plano, TX, USA] or needle for Seldinger technique. A small incision was made using a scalpel after local anesthetics before drain insertion. One-step technique was often preferred. In cases where the one-step technique was not appropriate (minor PE) or unsuccessful due to narrow intercostal space or thick parietal pleura, Seldinger technique was used.
The drains were secured using the pigtail thread lock and dressing according to the instructions of the manufacturer (Drain Fix, ConvaTec, Solna, Sweden). A urinary bag (different brands depending on date of insertion) was connected to the drain. Passive drainage was started immediately and initial volume drained was decided by the radiologist performing the insertion or the caring physician.
Data collection
Data were collected by one of the authors (S.S. or F.K.), who were resident radiologists, from the Department of Radiology’s administrative system for each drain on: insertion date and site, bore size, seniority of the radiologist (resident or specialist), and insertion technique (one-step or Seldinger).
Data were collected from the patient’s electronic medical records from the date of drain insertion to date of hospital discharge on: referring clinic, patient age and sex, drain removal date, effusion type [organ failure (e.g., heart or kidney), malignant, parapneumonic, empyema, other (e.g., hemothorax, pleuritis, post-surgery or pancreatitis), or unknown], and potential complications [blockage, dislocation, infection, misplacement, surgery or new drain (any size, within 2 weeks of study drain removal)]. Parapneumonic effusion was defined as simple PE without any finding of empyema on the same side as a diagnosed pulmonary infection. Drain blockage was defined as documented stopped drainage through the very small chest drain despite remaining PE without signs of drain misplacement or dislodgement. Repeat intervention was defined as surgery or the need for new drain (within 2 weeks of removing the study drain).
The etiology of PE was classified as the recorded diagnosis of the patient’s caring physician at the time of drain insertion. If there was no stated diagnosis but the case was clear on account of other data in the journals (e.g., malignant cells in PE on subsequent pathology report) the decision was made by the author who performed the data collection. If uncertainty still existed regarding the etiology of PE, the electronic medical records were screened during the three months after the date of the drain insertion. Difficult cases were discussed with the senior author (M.E.) who is a senior consultant in respiratory medicine.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical consent was approved by the Swedish National Ethics Review Board (No. 2020-07028). Individual consent was waived due to the use of already collected, pseudo-anonymized, administrative and medical records data.
Statistical analyses
Statistical analyses were performed using STATA version 14.2 (College Station, Texas, USA). Drain characteristics were presented using descriptive statistics. No data were imputed. As there were few 7 and 10 F drains, drain size was categorized as 6 or 7–10 F in the analyses. The risks of chest drain complications (for having at least one complication, and for each complication separately) were analyzed using multivariable logistic regression. Estimates were presented as adjusted odds ratios (aORs) with 95% confidence intervals (CIs), accounting for etiology of the PE, patient age, patient sex, radiologist seniority, and drain size. The statistical analyses were clustered by patient, to account for repeated drains in the same individual.
Results
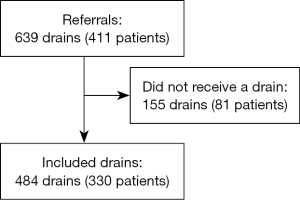
A total number of 484 very small chest drains, inserted in 330 patients, were included (Figure 1). The most common indication for drain insertion was malignant PE with 198 (40.9%) drains, followed by organ failure (n=105; 21.7%) (Table 1). Most drain insertions were referred from internal medicine (54.3%) and general surgery (16.7%). Mean age of patients was 74.6 years [standard deviation (SD) 11.9 years; range, 18–98 years], and 52.9% were men. Most drains (70.7%) were inserted by a specialist and 84.9% were inserted using one-step technique. The median time with drain was 3 days [interquartile range (IQR), 2–4 days; range, 0–16 days]. Main drain sizes were 6 F (73.3%) and 8 F (21.3%). Bore size was missing in 6 drains, which were excluded from further analysis.
Table 1
| Factor | Total (N=484) |
Organ failure (N=105) | Malignant (N=198) | Parapneumonic (N=34) | Empyema (N=40) | Other (N=45) |
Unknown (N=62) |
|---|---|---|---|---|---|---|---|
| Referring clinic | |||||||
| Thorax | 40 (8.3) | 25 (23.8) | 2 (1.0) | 2 (5.9) | 2 (5.0) | 8 (17.8) | 1 (1.6) |
| General surgery | 81 (16.7) | 6 (5.7) | 40 (20.2) | 1 (2.9) | 2 (5.0) | 15 (33.3) | 17 (27.4) |
| Gynecology | 30 (6.2) | 1 (1.0) | 26 (13.1) | 0 | 0 | 0 | 3 (4.8) |
| Infectious diseases | 49 (10.1) | 5 (4.8) | 3 (1.5) | 10 (29.4) | 25 (62.5) | 1 (2.2) | 5 (8.1) |
| Internal medicine | 263 (54.3) | 65 (61.9) | 115 (58.1) | 21 (61.8) | 11 (27.5) | 19 (42.2) | 32 (51.6) |
| Orthopedics | 8 (1.7) | 3 (2.9) | 0 | 0 | 0 | 1 (2.2) | 4 (6.5) |
| Palliative care | 8 (1.7) | 0 | 8 (4.0) | 0 | 0 | 0 | 0 |
| Unknown | 5 (1.0) | 0 | 4 (2.0) | 0 | 0 | 1 (2.2) | 0 |
| Age of the patient (years) | 74.6±11.9 | 79.9±9.5 | 72.6±11.2 | 75.5±15.1 | 73.0±12.1 | 69.8±13.9 | 75.9±10.7 |
| Sex of the patient | |||||||
| Female | 228 (47.1) | 39 (37.1) | 112 (56.6) | 13 (38.2) | 19 (47.5) | 15 (33.3) | 30 (48.4) |
| Male | 256 (52.9) | 66 (62.9) | 86 (43.4) | 21 (61.8) | 21 (52.5) | 30 (66.7) | 32 (51.6) |
| Seniority | |||||||
| Resident | 142 (29.3) | 33 (31.4) | 56 (28.3) | 15 (44.1) | 13 (32.5) | 14 (31.1) | 11 (17.7) |
| Specialist | 342 (70.7) | 72 (68.6) | 142 (71.7) | 19 (55.9) | 27 (67.5) | 31 (68.9) | 51 (82.3) |
| Insertion technique | |||||||
| One-step | 411 (84.9) | 98 (93.3) | 160 (80.8) | 30 (88.2) | 33 (82.5) | 40 (88.9) | 50 (80.6) |
| Seldinger | 73 (15.1) | 7 (6.7) | 38 (19.2) | 4 (11.8) | 7 (17.5) | 5 (11.1) | 12 (19.4) |
| Drain size (French) | |||||||
| 6 | 355 (73.3) | 91 (86.7) | 139 (70.2) | 27 (79.4) | 15 (37.5) | 32 (71.1) | 51 (82.3) |
| 7 | 5 (1.0) | 1 (1.0) | 1 (0.5) | 0 | 0 | 2 (4.4) | 1 (1.6) |
| 8 | 103 (21.3) | 13 (12.4) | 44 (22.2) | 5 (14.7) | 21 (52.5) | 11 (24.4) | 9 (14.5) |
| 10 | 15 (3.1) | 0 | 12 (6.1) | 0 | 3 (7.5) | 0 | 0 |
| Missing | 6 (1.2) | 0 | 2 (1.0) | 2 (5.9) | 1 (2.5) | 0 | 1 (1.6) |
| Days with drain | 3.5±2.5 | 3.1±2.1 | 4.2±3.0 | 2.6±1.7 | 3.6±1.8 | 2.3±2.1 | 3.0±1.7 |
| Missing | 3 (0.6) | 1 (1.0) | 2 (1.0) | 0 | 0 | 0 | 0 |
Data are presented as n (%) or mean ± SD. Thorax, department of cardiology and thoracic surgery; SD, standard deviation.
In total 192/478 (40.2%) of very small chest drains had at least one complication, translating into 43.3% of the patients. Multivariable analyses of the outcomes are shown in Table 2. Compared to PE due to organ failure (26.7%; aOR 1.0), the rate of any complication was increased in malignant PE (46.4%; aOR 2.5; 95% CI: 1.5–4.4) and empyema (71.8%; aOR 7.3; 95% CI: 2.9–18.6) (Table 2). There was only one infection related to drain insertion (in a patient with malignant PE) and there were no recorded cases of drain misplacement.
Table 2
| Factor | Any complication | Blockage | Dislodgement | Surgery | New drain | Repeat intervention (surgery or new drain) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate | aOR (95% CI) | Rate | aOR (95% CI) | Rate | aOR (95% CI) | Rate | aOR (95% CI) | Rate | aOR (95% CI) | Rate | aOR (95% CI) | ||||||
| Etiology of PE | |||||||||||||||||
| All | 192/478 (40.2) | 71/478 (14.9) | 79/478 (16.5) | 11/478 (2.3) | 98/478 (20.5) | 103/478 (21.5) | |||||||||||
| Organ failure | 28/105 (26.7) | 1 (ref) | 4/105 (3.8) | 1 (ref) | 16/105 (15.2) | 1 (ref) | 0/105 (0.0) | 1 (ref) | 10/105 (9.5) | 1 (ref) | 10/105 (9.5) | 1 (ref) | |||||
| Parapneumonic | 10/32 (31.3) | 1.3 (0.5–3.5) | 2/32 (6.3) | 1.9 (0.32–10.7) | 6/32 (18.8) | 1.4 (0.4–4.4) | 0/32 (0.0) | – | 4/32 (12.5) | 1.5 (0.4–5.7) | 4/32 (12.5) | 1.5 (0.4–5.6) | |||||
| Other | 11/45 (24.4) | 1.0 (0.4–2.5) | 3/45 (6.7) | 1.8 (0.3–9.6) | 1/45 (2.2) | 0.2 (0.0–1.2) | 2/45 (4.4) | Omitted (too few) |
7/45 (15.6) | 2.0 (0.7–5.6) | 8/45 (17.8) | 2.2 (0.8–6.3) | |||||
| Unknown | 24/61 (39.3) | 1.8 (0.9–3.6) | 7/61 (11.5) | 2.9 (0.7–11.8) | 13/61 (21.3) | 1.7 (0.7–3.9) | 0/61 (0.0) | – | 9/61 (14.8) | 1.7 (0.7–4.1) | 9/61 (14.8) | 1.6 (0.7–4.0) | |||||
| Malignant | 91/196 (46.4) | 2.5 (1.5–4.4) | 42/196 (21.4) | 6.3 (1.9–21.2) | 34/196 (17.3) | 1.4 (0.7–2.9) | 0/196 (0.0) | – | 50/196 (25.5) | 3.5 (1.7–7.2) | 50/196 (25.5) | 3.3 (1.6–6.8) | |||||
| Empyema | 28/39 (71.8) | 7.3 (2.9–18.6) | 13/39 (33.3) | 11.6 (3.0–44.8) | 9/39 (23.0) | 2 (0.7–5.8) | 9/39 (23.0) | 10.6 (1.4–79.4) | 18/39 (46.2) | 8.4 (3.4–20.8) | 22/39 (56.4) | 11.9 (4.8–29.4) | |||||
| Age (per 1 year increase) | 75.1±10.3 | 1.0 (1.0–1.0) | 73.4±10.3 | 1.0 (1.0–1.0) | 76.8±8.9 | 1.0 (1.0–1.0) | 65.2±9.0 | 0.9 (0.9–0.99) | 74.9±11.1 | 1 (1.0–1.0) | 74.4±11.3 | 1.0 (1.0–1.0) | |||||
| Sex | |||||||||||||||||
| Female | 98/227 (43.2) | 1 (ref) | 42/227 (18.5) | 1 (ref) | 37/227 (16.3) | 1 (ref) | 5/227 (2.2) | 1 (ref) | 50/227 (22.0) | 1 (ref) | 53/227 (23.3) | 1 (ref) | |||||
| Male | 94/251 (37.5) | 0.9 (0.6–1.4) | 29/251 (11.6) | 0.7 (0.4–1.2) | 42/251 (16.7) | 1.7 (0.7–1.9) | 6/251 (2.4) | 0.9 (0.2–4.5) | 48/251 (19.1) | 1.0 (0.6–1.6) | 50/251 (19.9) | 0.9 (0.5–1.6) | |||||
| Seniority | |||||||||||||||||
| Resident | 54/142 (38.0) | 1 (ref) | 14/142 (9.9) | 1 (ref) | 25/142 (17.6) | 1 (ref) | 5/142 (3.5) | 1 (ref) | 26/142 (18.3) | 1 (ref) | 27/142 (19.0) | 1 (ref) | |||||
| Specialist | 138/336 (41.1) | 1.2 (0.7–1.8) | 57/336 (17.0) | 1.9 (1.0–3.7) | 54/336 (16.1) | 0.9 (0.5–1.5) | 6/336 (1.8) | 0.4 (0.1–1.8) | 72/336 (21.4) | 1.3 (0.8–2.2) | 76/336 (22.6) | 1.3 (0.8–2.3) | |||||
| Drain size (French) | |||||||||||||||||
| 7–10 | 58/123 (47.2) | 1 (ref) | 25/123 (20.3) | 1 (ref) | 20/123 (16.2) | 1 (ref) | 6/123 (4.9) | 1 (ref) | 33/123 (26.8) | 1 (ref) | 37/123 (30.0) | 1 (ref) | |||||
| 6 | 134/355 (37.7) | 0.9 (0.5–1.4) | 46/355 (13.0) | 0.8 (0.5–1.4) | 59/355 (16.6) | 1.1 (0.6–1.9) | 5/355 (1.4) | 1. (0.3–7.6) | 65/355 (18.3) | 0.8 (0.5–1.4) | 66/355 (18.6) | 0.8 (0.5–1.3) | |||||
Data are presented as n/N (%) or mean ± SD. Associations with risk of complications analyzed using multivariable logistic regression, expressed as aOR. Each aOR is adjusted for all the other factors in the table. aOR, adjusted odds ratio; CI, confidence interval; PE, pleural effusion; ref, reference category; SD, standard deviation.
The most common complication overall was need of a new drain at 20.5% (Table 2). Compared to in organ failure, malignant PE was associated with significantly higher rates of blockage (21.4%; aOR 6.3; 95% CI: 1.9–21.2) and need of a new drain (25.5%; aOR 3.5; 95% CI: 1.7–7.2), and the risks were even higher in empyema (blockage: 33.3%; aOR 11.6; 95% CI: 3.0–44.8; and new drain: 46.2%; aOR 8.4; 95% CI: 3.4–20.8). Surgery as complication only occurred in empyema (23.0%; aOR 10.6; 95% CI: 1.4–79.4) and the group other PEs (4.4%, Table 2). The rate of repeat intervention was significantly higher in malignant PE (25.5%; aOR 3.3; 95% CI: 1.6–6.8) and empyema (56.4%; aOR 11.9; 95% CI: 4.8–29.4). The rate of repeat intervention was low in simple PE secondary to organ failure (9.5%) and for parapneumonic PE (12.5%). Mean age was significantly lower (65.2 years; aOR 0.9; 95% CI: 0.9–0.99) in patients who had surgery as complication.
Compared with residents, specialists had a higher rate of blockage as complication (17.0% vs. 9.9%; aOR 1.9; 95% CI: 1.0–3.7). There was no significant difference by patient sex or drain size for any complication (Table 2). There was no evidence of patient death directly related to drain insertion.
Discussion
Main findings
In this large, consecutive cohort study of 484 very small chest drains (<10 F), almost half of the drains were inserted to treat malignant PEs with a short-term failure rate of 25.5%. Compared to PEs due to organ failure, the odds of any complication was increased in malignant PE by 2.5 times (95% CI: 1.5–4.4) and in empyema by 7.3 times (95% CI: 2.9–18.6).
In empyema, the rate of surgery in our patients was 23.0%, which is close to the 19% reported by Rahman et al. (16). However, they did not report need of new drainage procedure other than surgery. In our study, most small-bore chest drains inserted for empyema (56.4%) later needed a new drain or surgery (within 2 weeks of the first drain). This is comparable to a previous report by Wozniak et al. on the use of pigtail catheters (size not specified) with a failure rate of 60% (defined as need of new drainage procedure or death) (17). Both the study by Rahman and Wozniak had similar failure rates with small versus large bore drains (16,17). The treatment and staging of empyema are complex and previous studies have shown that surgery have a superior outcome compared to chest drains in advanced empyema (4,18).
Dislodgement was observed in 16.5% of the drains. One contributing factor might be that these small chest drains are not routinely sutured. Two previous studies have reported a dislodgement rate of small bore chest drains of 6% and 21% (22,26).
Drain blockage was most common in empyema, occurring in one third (33.3%) of the drains which is within the previously reported range of 11–62% (4). One reason for our high rate of blockage in empyema could be that at the time of this study there was no hospital-wide routine for flushing small chest drains, and it was up to the separate wards to decide if and how this was done. The need for routine flushing of small chest drains is a potential confounder when comparing outcomes of small versus larger drains. The reason specialists had a higher rate of blockage compared to residents might be that they handle the more difficult clinical cases.
The preference in our study for one-step technique differs from what is recommended in guidelines, as they recommend that Selinger technique should be used primarily (9). One-step technique was used as it is easier to learn, faster, and considered safe as the use of ultrasound guidance allow imaging of the trocar tip during insertion.
Strengths and limitations
This study included all chest drains inserted at the Department during the study period, and the Blekinge Hospital is the only hospital in the region. As only very small-bore chest drains (6–10 F) were inserted, there was no selection bias in the choice of drainage compared to larger drains. Another strength of this study is that all radiologists routinely insert drains with a similar technique using ultrasound guidance which helps in standardizing and generalizing the findings. The wide inclusion criteria imply a broader application of our results as patients with PEs often are very ill with a wide combination of underlying conditions. The multivariable analysis allowed comparison of repeat intervention and complications accounting for differences in PE etiology, patient age and sex, drain size, radiologist seniority.
A limitation with retrospective studies is the uncertainty of how the collected data was conceived (including findings on pleural ultrasound). For example, cases are sometimes without a clear etiology at the time of chest drain insertion and we cannot know for sure that clinicians followed the same diagnostic criteria. It is difficult in retrospect to analyse in detail when a diagnosis is set because of the wide difference in how clinicians practice medicine and document their workflow. The inclusion of definitive diagnosis set after drain insertion was an attempt to alleviate this, however it might make the results harder to apply in a clinical setting. The observational design and lack of a comparable group treated with larger-bore drains prevents a direct comparison of outcomes. Data on pneumothorax, pleural fibrinolysis, or pleurodesis were not evaluated. In our hospital there is no routine imaging made after drain insertion so the results would be prone to bias and was thus not investigated. Mortality rate was outside the scope of this study as the absence of a control group would have made the results difficult to interpret.
Clinical implications
For the clinician, a key finding is that very small chest drains (6–10 F) was often insufficient for the treatment of empyema, with increased rates of complications and repeat intervention compared to in other types of PE, and additional drains or surgery for empyema was frequently needed. These findings support the need of larger-bore drains and early consultation with a thoracic surgeon when treating patients with empyema as to not prolong time to definitive treatment. Pleural ultrasound can inform the need and type of drainage, with echogenic pleural fluid or septa indicating an increased risk of complications when using very small chest drains and the need for larger drains (such as 12 F or larger) (15). Small chest drains should be routinely flushed (15). Identifying suspected empyema versus likely non-empyema PE at the time of diagnosis is essential to guide appropriate type of chest drain for effective treatment. A very small-bore chest drain was successful in the vast number of cases of simple PE, but the relatively high rate of drain dislodgement suggested the need to fixate and secure the very small drains more effectively such as through drain suture.
Conclusions
A single small-bore chest drain (6–10 F) was associated with low rates of complications in simple PEs, but showed high rates of complications in empyema, with the frequent need of additional drains or surgery. These findings support use of larger drains and early consultation with a thoracic surgeon in empyema.
Acknowledgments
The authors thank Dr. Malin Cullin, senior consultant in radiology at Blekinge Hospital, for clinical insights and supervision.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1457/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1457/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1457/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1457/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical consent was approved by the Swedish National Ethics Review Board (No. 2020-07028). Individual consent was waived due to the use of already collected, pseudo-anonymized, administrative and medical records data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Light RW. Pleural effusions. Med Clin North Am 2011;95:1055-70. [Crossref] [PubMed]
- Goligher EC, Leis JA, Fowler RA, et al. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care 2011;15:R46. [Crossref] [PubMed]
- Cartaxo AM, Vargas FS, Salge JM, et al. Improvements in the 6-min walk test and spirometry following thoracentesis for symptomatic pleural effusions. Chest 2011;139:1424-9. [Crossref] [PubMed]
- Shen KR, Bribriesco A, Crabtree T, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg 2017;153:e129-46. [Crossref] [PubMed]
- Hooper C, Maskell N. BTS audit team. British Thoracic Society national pleural procedures audit 2010. Thorax 2011;66:636-7. [Crossref] [PubMed]
- Miller KS, Sahn SA. Chest tubes. Indications, technique, management and complications. Chest 1987;91:258-64. [Crossref] [PubMed]
- Gammie JS, Banks MC, Fuhrman CR, et al. The pigtail catheter for pleural drainage: a less invasive alternative to tube thoracostomy. JSLS 1999;3:57-61.
- Clementsen P, Evald T, Grode G, et al. Treatment of malignant pleural effusion: pleurodesis using a small percutaneous catheter. A prospective randomized study. Respir Med 1998;92:593-6. [Crossref] [PubMed]
- Havelock T, Teoh R, Laws D, et al. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii61-76. [Crossref] [PubMed]
- Keeling AN, Leong S, Logan PM, et al. Empyema and effusion: outcome of image-guided small-bore catheter drainage. Cardiovasc Intervent Radiol 2008;31:135-41. [Crossref] [PubMed]
- Akhan O, Ozkan O, Akinci D, et al. Image-guided catheter drainage of infected pleural effusions. Diagn Interv Radiol 2007;13:204-9.
- Cantin L, Chartrand-Lefebvre C, Lepanto L, et al. Chest tube drainage under radiological guidance for pleural effusion and pneumothorax in a tertiary care university teaching hospital: Review of 51 cases. Can Respir J 2005;12:29-33. [Crossref] [PubMed]
- Sartori S, Tombesi P, Tassinari D, et al. Sonographically guided small-bore chest tubes and sonographic monitoring for rapid sclerotherapy of recurrent malignant pleural effusions. J Ultrasound Med 2004;23:1171-6. [Crossref] [PubMed]
- Kiranantawat N, Sungsiri J, Geater SL. Outcome of ultrasound-guided small-bore catheter drainage in exudative pleural effusions. J Med Assoc Thai 2014;97:548-53.
- Roberts ME, Rahman NM, Maskell NA, et al. British Thoracic Society Guideline for pleural disease. Thorax 2023;78:s1-s42. [Crossref] [PubMed]
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010;137:536-43. [Crossref] [PubMed]
- Wozniak CJ, Paull DE, Moezzi JE, et al. Choice of first intervention is related to outcomes in the management of empyema. Ann Thorac Surg 2009;87:1525-30; discussion 1530-1. [Crossref] [PubMed]
- Bedawi EO, Ricciardi S, Hassan M, et al. ERS/ESTS statement on the management of pleural infection in adults. Eur Respir J 2023;61:2201062. [Crossref] [PubMed]
- Thethi I, Ramirez S, Shen W, et al. Effect of chest tube size on pleurodesis efficacy in malignant pleural effusion: a meta-analysis of randomized controlled trials. J Thorac Dis 2018;10:355-62. [Crossref] [PubMed]
- Parulekar W, Di Primio G, Matzinger F, et al. Use of small-bore vs large-bore chest tubes for treatment of malignant pleural effusions. Chest 2001;120:19-25. [Crossref] [PubMed]
- Chetty GK, Battula NR, Govindaswamy R, et al. Comparative analysis of the Bonanno catheter and tube thorocostomy in effective aspiration of pleural effusion. Heart Surg Forum 2006;9:E731-4. [Crossref] [PubMed]
- Horsley A, Jones L, White J, et al. Efficacy and complications of small-bore, wire-guided chest drains. Chest 2006;130:1857-63. [Crossref] [PubMed]
- Kulvatunyou N, Erickson L, Vijayasekaran A, et al. Randomized clinical trial of pigtail catheter versus chest tube in injured patients with uncomplicated traumatic pneumothorax. Br J Surg 2014;101:17-22. [Crossref] [PubMed]
- Rahman NM, Pepperell J, Rehal S, et al. Effect of Opioids vs NSAIDs and Larger vs Smaller Chest Tube Size on Pain Control and Pleurodesis Efficacy Among Patients With Malignant Pleural Effusion: The TIME1 Randomized Clinical Trial. JAMA 2015;314:2641-53. [Crossref] [PubMed]
- Statistics sweden(statistikmyndigheten). Folkmängd i landskapen den 31 december 2021. Published online March 22, 2022. Accessed January 19, 2023. Available online: https://www.scb.se/hitta-statistik/statistik-efter-amne/befolkning/befolkningens-sammansattning/befolkningsstatistik/pong/tabell-och-diagram/folkmangd-och-befolkningsforandringar---helarsstatistik/folkmangd-i-landskapen-den-31-december-2021/
- Davies HE, Merchant S, McGown A. A study of the complications of small bore 'Seldinger' intercostal chest drains. Respirology 2008;13:603-7. [Crossref] [PubMed]