
Patients with idiopathic pulmonary fibrosis and refractory cough have traction bronchiectasis and distorted airway architecture: a retrospective case review study
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive interstitial lung disease of unknown cause. Although the clinical course of IPF varies for each patient, the prognosis of IPF is generally poor. The median survival time after diagnosis ranges from 2 to 3 years (1). The primary aims of treatment for patients with IPF is to delay disease progression, alleviate the coughing, and improve quality of life (QOL) (2). Antifibrotic drugs such as pirfenidone and nintedanib have been found to reduce decreases in lung function, mortality rates, and risks of acute exacerbation (3-9).
A chronic dry cough and dyspnea on exertion are among the main signs/symptoms of IPF. Although the prevalence of cough in patients with IPF varies, some studies have found that up to 80% patients with IPF complained of cough (10,11). Cough in IPF patients is generally severe and difficult to control, and can adversely impact the activities of daily life (12). Furthermore, a previous study has reported that cough might be an independent predictor of patient outcome (10). Although mechanisms involved in the cough of IPF patients have been proposed, the exact pathophysiology and appropriate treatments for these patients remain unclear (13-16). To clarify the mechanisms of cough in IPF patients, we retrospectively reviewed the patients diagnosed with IPF at our hospital and compared the clinical features of IPF patients with refractory cough with the clinical features of IPF patients without refractory cough.
Methods
We retrospectively reviewed the records of all patients who visited the Department of Respiratory Medicine, Kanazawa University Hospital between January 2008 and August 2016. Clinical data were collected from medical records. Those patients who satisfied the established criteria for the diagnosis of IPF were included in the study (17). High-resolution computed tomography (HRCT) findings were analyzed by two radiologists. Specifically, one of the two radiologists analyzed the HRCT images, and the other radiologist verified the analysis of the first radiologist. Lung specimens were evaluated in a similar manner by two pathologists. We defined HRCT findings as follows: honeycombing, clusters of cystic airspaces just below the pleura; emphysema, abnormal enlargement of the airspaces distal to the terminal bronchioles plus destruction of alveolar walls; and traction bronchiectasis, bronchoarterial ratio greater than 1.0 and lack of tapering. Pulmonary function testing was performed by a computerized spirometer (CHESTAC-9800; CHEST, Tokyo, Japan). Fractional exhaled nitric oxide (FeNO) was measured by an electrochemical analyzer (NA623NP; CHEST). Assessment of capsaicin cough sensitivity was carried out by a previously reported method (18).
We investigated the prevalence of refractory cough in patients with IPF and compared the clinical features of the patients with refractory cough with those of the patients without refractory cough. At every clinic visit we asked each patient about the presence of cough, even if cough was not observed during the visit. We performed the following investigations: blood tests; radiography of the chest and sinuses; chest computed tomography (CT); spirometry; bronchodilator reversibility test; FeNO test; and on patients with cough, the capsaicin cough sensitivity test. The results of the investigations allowed us to identify patients with comorbidities that were more likely to cause cough, such as bronchial asthma (BA), cough-variant asthma (CVA), atopic cough (AC), and sinobronchial syndrome (SBS), and treated each of those patients specifically for his or her concomitant disease (e.g., inhaled corticosteroids for BA, bronchodilators for CVA, histamine H1 receptor antagonists for AC, and macrolides and expectorants for SBS). In this study, cough due to IPF was defined as a cough that persisted despite complete evaluations and treatments for comorbidities that cause cough, which were based on published practice guidelines (19).
Continuous variables, excluding capsaicin cough sensitivity, are shown as mean ± standard deviation (SD). Capsaicin cough sensitivity is expressed as a geometric mean with a geometric standard error of the mean. Discrete variables are shown as numbers. Statistical differences between pairs of groups were determined by the Mann-Whitney U test or Fisher exact test. A P value less than 0.05 was considered statistically significant. All statistical analyses were performed by EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan) (20), which is a graphical user interface based on a modified version of R commander (The R Foundation for Statistical Computing, Vienna, Austria). The modified version of R commander has additional statistical functions that are frequently used in biostatistical analysis.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethical review board of Kanazawa University Hospital (approval date: February 20, 2019; approval No. 2960) and individual consent for this retrospective analysis was waived.
Results
Figure 1 shows the numbers and types of patients evaluated in this study. Among a total of 23 patients with IPF, 10 (43.5%) patients had chronic cough lasting longer than 8 weeks. Of the ten patients, seven patients had concomitant conditions that could lead to cough (two patients with BA, two with CVA, three with AC, and two with SBS). Of these seven patients, the cough of four patients was resolved after treatment of their concomitant condition. The cough of the remaining three of seven patients could not be stopped by specific treatments targeting their concomitant condition. Finally, among the 23 patients there were 6 (26.1%) with refractory cough associated with IPF.
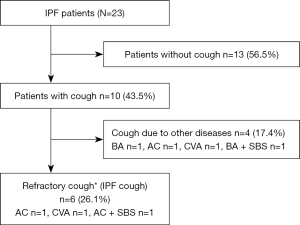
Table 1 compares the characteristics, laboratory data, and HRCT findings at the diagnosis of IPF patients with refractory cough and of IPF patients without cough. The body mass index (BMI) of patients with cough was significantly lower than the BMI of patients without cough (18.8±2.5 vs. 22.8±2.5 kg/m2, respectively; P<0.01). More patients with cough than patients without cough received antifibrotic drugs, especially pirfenidone. None of the patients received angiotensin-converting enzyme (ACE) inhibitors. IPF disease severity was evaluated according to the GAP {gender (G), age (A), and two lung physiology variables [P; forced vital capacity (FVC) and diffusing capacity of carbon monoxide (DLco)]} index (21), and the difference between patients with and without cough was not significant.
Table 1
| Variables | With refractory cough (n=6) | Without refractory cough (n=17) | P value |
|---|---|---|---|
| Age, years | 60 [38–79] | 67 [55–79] | 0.19 |
| Sex | >0.99 | ||
| Male | 5 (83.3) | 14 (82.4) | |
| Female | 1 (16.7) | 3 (17.6) | |
| BMI, kg/m2 | 18.8±2.5 | 22.8±2.5 | <0.01 |
| Smoking status | >0.99 | ||
| Never smoker | 1 (16.7) | 2 (11.8) | |
| Current or former smoker | 5 (83.3) | 15 (88.2) | |
| Medications | |||
| Antifibrotic drugs | 6 (100.0) | 9 (52.9) | 0.06 |
| Pirfenidone | 6 (100.0) | 7 (41.2) | 0.02 |
| Nintedanib | 4 (66.7) | 4 (23.5) | 0.13 |
| Prednisolone | 0 (0.0) | 3 (17.6) | 0.54 |
| Proton pump inhibitor | 4 (66.7) | 6 (35.3) | 0.34 |
| Expectorant | 1 (16.7) | 2 (11.8) | >0.99 |
| Peripheral blood eosinophil count, mm3 | 130.8±91.3 | 315.5±338.9 | 0.09 |
| Total serum IgE, IU/mL | 139.2±204.0 | 227.0±183.7 | 0.68 |
| KL-6, U/mL | 1,085.0±254.0 | 835.1±496.9 | 0.06 |
| GAP index | 0.82 | ||
| Stage I | 3 (50.0) | 11 (64.7) | |
| Stage II | 2 (33.3) | 4 (23.5) | |
| Stage III | 1 (16.7) | 2 (11.8) | |
| HRCT findings | |||
| Traction bronchiectasis | 5 (83.3) | 2 (11.8) | <0.01 |
| Honeycombing | 6 (100.0) | 15 (88.2) | >0.99 |
| Emphysema | 1 (16.7) | 8 (47.1) | 0.34 |
Data are presented as n (%), mean ± SD, or median [range]. IPF, idiopathic pulmonary fibrosis; N, total number of patients evaluated; HRCT, high-resolution computed tomography; n, number in subgroups; BMI, body mass index; IgE, immunoglobulin E; GAP, gender (G), age (A), and two lung physiology variables (P; FVC and DLco); FVC, forced vital capacity; DLco, diffusing capacity of carbon monoxide; SD, standard deviation.
Among the HRCT findings, the differences between the rates of honeycombing and emphysema were not significant for the patients with and without cough. Traction bronchiectasis was significantly more common in the patients with cough than in the patients without cough (five of six patients vs. two of 17 patients, respectively; P<0.01).
Figure 2 shows representative HRCT images of patients with refractory cough and patients without refractory cough. HRCT images of IPF patients with refractory cough show not only honeycombing, but also traction bronchiectasis and architectural distortion of the airways. HRCT images of IPF patients without refractory cough show almost normal bronchial architecture.

Table 2 compares the parameters of pulmonary function of IPF patients with refractory cough and the parameters of pulmonary function of IPF patients without refractory cough at diagnosis. The FVC of the patients with cough was significantly lower than the FVC of patients without cough (77.5%±30.4% predicted vs. 99.9%±0.5% predicted, respectively; P=0.046). The differences between the other measured parameters, including FeNO and capsaicin cough sensitivity, of the IPF patients with and without cough were not significant.
Table 2
| Parameters | With refractory cough (n=6) | Without refractory cough (n=17) | P value |
|---|---|---|---|
| FVC, % predicted | 77.5±30.4 | 99.9±0.5 | 0.046 |
| FEV1, % predicted | 85.8±28.2 | 96.7±24.7 | 0.52 |
| FRC, % predicted | 77.4±16.4 | 85.6±18.2 | 0.40 |
| RV, % predicted | 71.4±18.6 | 79.6±21.0 | 0.36 |
| TLC, % predicted | 72.3±22.5 | 87.9±19.1 | 0.25 |
| RV/TLC, % predicted | 102.3±25.1 | 90.9±15.9 | 0.40 |
| DLco, % predicted | 48.4±21.9 | 53.7±14.2 | 0.26 |
| FeNO, ppb | 19.0±6.0 | 29.2±16.7 | 0.29 |
| C5, µM (with cough n=5, without cough n=10) | 20.6±26.6 | 12.7±8.1 | 0.62 |
| Increased cough receptor sensitivity to capsaicin | 2/5 (40.0) | 1/10 (10.0) | 0.16 |
Data are presented as n (%), mean ± SD, or geometric mean ± geometric standard error. IPF, idiopathic pulmonary fibrosis; N, total number of patients evaluated; n, number in subgroups; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; FRC, functional residual capacity; RV, residual volume; TLC, total lung capacity; DLco, diffusing capacity of carbon monoxide; FeNO, fractional exhaled nitric oxide; ppb, parts per billion; C5, capsaicin concentration eliciting 5 or more coughs; SD, standard deviation.
Discussion
In this study, 6 (26.1%) of 23 IPF patients complained of refractory cough. We found that several clinical features of patients with IPF and refractory cough were different from the same features of IPF patients without refractory cough, as follows: lower BMI, lower FVC, and traction bronchiectasis and distorted airway architecture vs. almost normal airway architecture on HRCT scans.
Although the exact pathophysiology of cough in IPF patients remains unclear, the following mechanisms have been proposed: (I) increased sensitivity of the cough reflex; (II) mechanical stimulation; (III) accumulation of mucus in the airways; and (IV) comorbidities that cause cough (13-16). A previous study found that more than 50% of patients with interstitial lung disease also had other common diseases that are associated with cough (22). In our study, seven of ten IPF patients with cough had concomitant conditions associated with cough, and the cough of four of seven patients was resolved by specific treatments for each condition. On the other hand, three patients had no other possible causes of cough, and it is clear that IPF alone can cause cough by pathophysiological mechanisms thought to be associated with IPF.
There have been a few reports that the sensitivity of the cough reflex to chemical irritants is increased in patients with IPF (11,23). One of the published reports on patients with IPF examined the sensitivity of the cough reflex to capsaicin; patients with comorbidities associated with cough such as BA, gastroesophageal reflex disease (GERD), respiratory tract infections, and ACE inhibitors were excluded (11). However, patients with other conditions, in particular AC, may not have been completely excluded because of an incomplete history on specific preventative treatments such as inhaled corticosteroids and histamine H1 receptor antagonists. Indeed, cough has been dramatically reduced by steroid therapy, which is not recommended for IPF patients because it is not effective; and AC might have been a concomitant condition in some studies (11). In our study, the sensitivity of the cough reflex to capsaicin was only increased in three patients (two patients with refractory cough and one without cough), and the difference between the sensitivity of the cough reflex to capsaicin in the two groups of patients was not significant. However, a capsaicin cough challenge test was assessed for only 15 of 23 patients (five patients with cough and ten patients without cough). Additional studies are needed to clarify the association of the sensitivity of the cough reflex to capsaicin in IPF patients.
Mechanical stimulation is one of the proposed causes of cough in IPF patients. A previous study found that the sensitivity of the cough reflex to mechanical stimuli is enhanced in IPF patients (24). Furthermore, mechanical stimulation of the base of the posterior lung, where fibrotic changes are most prevalent and extensive in IPF patients, more frequently induced cough than mechanical stimulation of other regions of the lung (24). Additionally, Sato et al. showed that the inhibition of mechanical stimulation of the thoracic cage by a chest band reduced the frequency of cough due to mechanical stimulation (25). Our study found that traction bronchiectasis and distorted airway architecture of the airways were specific features of patients with IPF and refractory cough. However, the differences between the rates of honeycombing and emphysema in our study patients with and without refractory cough were not significant.
Previous studies have shown that bronchopulmonary C-fibers and Aδ-fibers play an important role in the cough reflex (26,27). C-fibers, which densely innervate the epithelium and the region around the epithelium of whole airways, are sensitive to a diverse range of chemical and environmental irritants.
On the other hand, Aδ-fibers, which sparsely innervate the space between the epithelium and smooth muscle in the proximal airways, are insensitive to most chemical irritants, but are sensitive to mechanical stimuli. The distribution of sensory nerves combined with our study findings, suggest that architectural distortion of the bronchi exerts mechanical stress on the airways and leads to increased stimulation of the Aδ-fibers, which results in cough. To our best knowledge, there have been no reports on investigations of the association between structural changes seen on HRCT and cough in IPF patients.
Although patients with IPF commonly have a nonproductive dry cough, accumulation of mucus in the airways is thought to be another cause of cough. A previous report showed that a common polymorphism in the promoter of the mucin 5B gene (MUC5B) is associated with the development of IPF and increased production of airway mucin (28). Indeed, mucin 5B protein is the predominant component of the honeycomb cysts of IPF patients (29). Furthermore, the severity of cough in IPF patients is associated with this MUC5B genotype (30). These reports suggest that increased MUC5B-associated mucus may contribute to the cough in IPF patients. In our study, all patients with refractory cough had a dry cough, and the association between accumulation of mucus in the airways and cough in IPF patients could not be evaluated. Additional studies are needed to clarify the association of the accumulation of mucus in the airways with cough in IPF patients.
In our study, the BMI and FVC were significantly lower in IPF patients with refractory cough than in IPF patients without cough. Because the cough of IPF patients is debilitating and persistent, the BMI of patients with IPF might have been decreased because of prolonged repeated episodes of cough. Furthermore, we believe that the decreased FVC was due to the extensive fibrosis revealed on HRCT.
There are no established treatments for cough in IPF patients. Antifibrotic drugs have been shown to reduce decreases in lung function and improve the survival of IPF patients (3-9). Some studies found that pirfenidone reduced the rates of cough in IPF patients (31,32). We previously showed that pirfenidone suppressed the increased sensitivity of the cough reflex due to capsaicin in a sensitized guinea pig model (33). Almost all the major clinical trials of antifibrotic drugs have not focused on cough, and the efficacy of these drugs on cough is unclear. All our study patients with refractory cough were treated with antifibrotic drugs. Additionally, trials studying a P2X3 receptor antagonist, which has recently demonstrated efficacy in reducing the rates of cough and improved the QOL of patients with refractory chronic cough or unexplained chronic cough (34), found that the P2X3 receptor antagonist also tended to reduce the rates of cough in IPF patients. However, the primary endpoint was not met in the trials (35). Further studies are needed to establish the management of cough in IPF patients.
Our study has several limitations. First, it was a single-center retrospective study of a limited number of patients. Second, the radiological findings were only subjectively evaluated by radiologists. An objective evaluation that includes software that can analyze images is preferable for confirming the relationship between structural changes on HRCT and cough in patients with IPF. However, the disease severity of IPF as assessed by GAP scoring did not show an association with cough. We speculate that mechanical stress due to distorted architecture of the airways may be the cause of cough in IPF patients, regardless of the extent or severity of structural changes. Third, some comorbidities associated with cough, especially GERD, may not have been adequately ruled out. GERD is a major problem that is often found in patients with IPF, and it is one of the main causes of chronic cough. There were no differences between the characteristics of the patients with cough (n=10) and without cough (n=13) (Tables S1,S2). Finally, we defined “refractory cough due to IPF” as a cough that persists despite complete evaluations and treatments for comorbidities that cause cough, which are based on published practice guidelines (19). We could not objectively evaluate the frequency and severity of cough with the use of established cough assessment tools. To clarify the exact mechanism for cough in IPF patients, we need additional studies that overcome these limitations.
Conclusions
We found that 26.1% of our patients with IPF complained of refractory cough. Our results suggest that mechanical stress due to distorted architecture of the airways is a major factor associated with cough in IPF patients. To clarify the mechanisms and establish the appropriate treatment strategy for cough in IPF patients, we need additional studies focusing on the cough of patients with IPF.
Acknowledgments
The authors are grateful to JAM Post Inc. for carefully proofreading the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was a standard submission to the series “Cough Section” published in Journal of Thoracic Disease. The article has undergone external peer review.
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1443/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1443/coif). The series “Cough Section” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethical review board of Kanazawa University Hospital (approval date: February 20, 2019; approval No. 2960) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431-40. [Crossref] [PubMed]
- Raghu G, Richeldi L. Current approaches to the management of idiopathic pulmonary fibrosis. Respir Med 2017;129:24-30. [Crossref] [PubMed]
- Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:1040-7. [Crossref] [PubMed]
- Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010;35:821-9. [Crossref] [PubMed]
- Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011;377:1760-9. [Crossref] [PubMed]
- King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083-92. [Crossref] [PubMed]
- Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med 2011;365:1079-87. [Crossref] [PubMed]
- Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071-82. [Crossref] [PubMed]
- Petnak T, Lertjitbanjong P, Thongprayoon C, et al. Impact of Antifibrotic Therapy on Mortality and Acute Exacerbation in Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. Chest 2021;160:1751-63. [Crossref] [PubMed]
- Ryerson CJ, Abbritti M, Ley B, et al. Cough predicts prognosis in idiopathic pulmonary fibrosis. Respirology 2011;16:969-75. [Crossref] [PubMed]
- Hope-Gill BD, Hilldrup S, Davies C, et al. A study of the cough reflex in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003;168:995-1002. [Crossref] [PubMed]
- Key AL, Holt K, Hamilton A, et al. Objective cough frequency in Idiopathic Pulmonary Fibrosis. Cough 2010;6:4. [Crossref] [PubMed]
- van Manen MJ, Birring SS, Vancheri C, et al. Cough in idiopathic pulmonary fibrosis. Eur Respir Rev 2016;25:278-86. [Crossref] [PubMed]
- Myall KJ, Kavanagh JE, Birring SS. Idiopathic pulmonary fibrosis-associated cough: Mechanisms and management. Pulm Pharmacol Ther 2019;56:100-3. [Crossref] [PubMed]
- Mann J, Goh NSL, Holland AE, et al. Cough in Idiopathic Pulmonary Fibrosis. Front Rehabil Sci 2021;2:751798. [Crossref] [PubMed]
- Liu S, Ye X. Assessment and Management of Cough in Idiopathic Pulmonary Fibrosis: A Narrative Review. Lung 2023;201:531-44. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Fujimura M, Sakamoto S, Kamio Y, et al. Effects of methacholine induced bronchoconstriction and procaterol induced bronchodilation on cough receptor sensitivity to inhaled capsaicin and tartaric acid. Thorax 1992;47:441-5. [Crossref] [PubMed]
- Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020;55:1901136. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012;156:684-91. [Crossref] [PubMed]
- Madison JM, Irwin RS. Chronic cough in adults with interstitial lung disease. Curr Opin Pulm Med 2005;11:412-6. [Crossref] [PubMed]
- Doherty MJ, Mister R, Pearson MG, et al. Capsaicin induced cough in cryptogenic fibrosing alveolitis. Thorax 2000;55:1028-32. [Crossref] [PubMed]
- Jones RM, Hilldrup S, Hope-Gill BD, et al. Mechanical induction of cough in Idiopathic Pulmonary Fibrosis. Cough 2011;7:2. [Crossref] [PubMed]
- Sato R, Handa T, Matsumoto H, et al. Antitussive Effect of a Chest Band in Patients with Interstitial Lung Disease: The Preliminary Results from a Pre-post Intervention Study. Intern Med 2021;60:3701-7. [Crossref] [PubMed]
- Narula M, McGovern AE, Yang SK, et al. Afferent neural pathways mediating cough in animals and humans. J Thorac Dis 2014;6:S712-9. [Crossref] [PubMed]
- Canning BJ, Chang AB, Bolser DC, et al. Anatomy and neurophysiology of cough: CHEST Guideline and Expert Panel report. Chest 2014;146:1633-48. [Crossref] [PubMed]
- Seibold MA, Wise AL, Speer MC, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 2011;364:1503-12. [Crossref] [PubMed]
- Seibold MA, Smith RW, Urbanek C, et al. The idiopathic pulmonary fibrosis honeycomb cyst contains a mucocilary pseudostratified epithelium. PLoS One 2013;8:e58658. [Crossref] [PubMed]
- Scholand MB, Wolff R, Crossno PF, et al. Severity of cough in idiopathic pulmonary fibrosis is associated with MUC5 B genotype. Cough 2014;10:3. [Crossref] [PubMed]
- Azuma A, Taguchi Y, Ogura T, et al. Exploratory analysis of a phase III trial of pirfenidone identifies a subpopulation of patients with idiopathic pulmonary fibrosis as benefiting from treatment. Respir Res 2011;12:143. [Crossref] [PubMed]
- van Manen MJG, Birring SS, Vancheri C, et al. Effect of pirfenidone on cough in patients with idiopathic pulmonary fibrosis. Eur Respir J 2017;50:1701157. [Crossref] [PubMed]
- Okazaki A, Ohkura N, Fujimura M, et al. Effects of pirfenidone on increased cough reflex sensitivity in guinea pigs. Pulm Pharmacol Ther 2013;26:603-8. [Crossref] [PubMed]
- McGarvey LP, Birring SS, Morice AH, et al. Efficacy and safety of gefapixant, a P2X(3) receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet 2022;399:909-23. [Crossref] [PubMed]
- Martinez FJ, Afzal AS, Smith JA, et al. Treatment of Persistent Cough in Subjects with Idiopathic Pulmonary Fibrosis (IPF) with Gefapixant, a P2X3 Antagonist, in a Randomized, Placebo-Controlled Clinical Trial. Pulm Ther 2021;7:471-86. [Crossref] [PubMed]


