Recommended approaches for screening and early detection of lung cancer in the Middle East and Africa (MEA) region: a consensus statement
Highlight box
Key recommendations
• Health authorities in the Middle East and Africa (MEA) region must be informed about the critical need for comprehensive lung cancer screening programs. Increasing public awareness about the risks, benefits, and accessibility of screening programs for high-risk groups can significantly improve survival rates.
• Campaigns emphasizing the risks of smoking targeting young people through education in schools and social media can effectively prevent smoking initiation, contributing to a healthier, smoke-free future generation.
What was recommended and what is new?
• Implementation of lung cancer screening programs in the MEA region remains significantly limited likely due to resource constraints, varying healthcare priorities, and a lack of widespread awareness about the benefits of early detection.
• The MEA region requires tailored lung cancer screening programs that consider local risk factors and cultural specifics. Establishing such programs faces challenges in integration with existing healthcare systems and securing necessary funding and expertise.
What is the implication, and what should change now?
• Research on risk factors, awareness programs for physicians and patients, and integration of lung cancer screening initiatives with tobacco control programs in the MEA region may facilitate higher adherence to lung cancer screening.
• Collaborative efforts for funding and resource allocation, alongside adapting strategies to local contexts, are essential to enhance early detection and improve overall cancer care outcomes in the region.
Introduction
Lung cancer is the second most commonly diagnosed cancer and the leading cause of cancer-related deaths globally, accounting for the greatest economic and public health burden of all cancers (1-3). In 2020, lung cancer accounted for approximately 2.2 million cases and nearly 1.8 million deaths worldwide (2,4). Although lung cancer has a multifactorial etiology with smoking being the major risk factor, it is not just a “smoker’s disease”. While individuals with a smoking history remain at high-risk even years after quitting smoking, lung cancer prevalence in individuals with no smoking history is gradually rising (5). While still the leading cause of morbidity and mortality among men, the incidence and mortality due to lung cancer is rising among women (2,6-9).
The prevalence of lung cancer in the Middle East and Africa (MEA) region has steadily increased in recent years, with non-small cell lung cancer (NSCLC) constituting approximately 85% of all detected cases; adenocarcinoma is the commonly diagnosed subtype (10-15). A high proportion of lung cancer cases are detected at an advanced stage in the MEA region resulting in poor prognosis with a 5-year survival rate of <10% and limited treatment options (3,10,13). Early detection through screening may affect patient outcomes with a considerable impact on quality of life and the economic toll (3,16,17). Some large-scale clinical trials have shown that targeted low-dose computerized tomography (LDCT) screening can significantly reduce lung cancer mortality in high-risk individuals by nearly a quarter (18,19). Expert opinion suggests screening for lung cancer compares favorably with other cancer screening programs in terms of costeffectiveness and potential benefits (3). Here we explore the factors leading to delayed diagnoses as well as the challenges and gaps in the early screening, detection, and referral framework of lung cancer in the MEA region and the possible role that may be played by artificial intelligence (AI) based screening techniques.
Methods
A steering committee meeting was convened in October 2022 to analyze the current unmet needs and challenges in the screening and early diagnosis of lung cancer in the MEA region. A panel comprising ten key external experts in the field of oncology from the Kingdom of Saudi Arabia (KSA), United Arab Emirates (UAE), South Africa, Egypt, Lebanon, Jordan, and Turkey congregated to discuss the disease burden, risk factors, and challenges in early screening, diagnosis, and management of lung cancer in the MEA region (Table S1). The panel reviewed the current guidelines for the screening of lung cancer, their implementation, and effectiveness in identifying early lung cancer cases. The panel also discussed the potential impact of using AI in lung cancer screening and early detection. The opinions and suggestions expressed in this consensus statement were reached after deliberations based on the panel members’ clinical expertise in lung cancer patient care, and applicable and relevant published evidence. In this report we primarily focus on information from the countries represented by the external experts attending the steering committee meeting. Very little information was available from other countries of the region and thus was excluded.
Epidemiology and burden of lung cancer in the MEA region
Although lung cancer is among the top 10 malignancies detected in the MEA region, accurate data on lung cancer incidence rates are not available due to a lack of updated population registries in several countries of the region (10,13). In 2018, the estimated number of new lung cancer cases in the MEA region was 79,887 (13). Lung cancer comprised 4.0% of 4,707 total cancer cases in UAE in 2018 and 8.8% of 2,079 cancer-related deaths out of a population of approximately 10 million (20). An epidemiological study reported that Lebanon had the highest incidence of lung cancer in the MEA region between 2005 and 2015, accounting for 9.2% of all new cancer cases, i.e., about 950 cases per year (21). Egypt reported an increase in the incidence of lung cancer between 1980 and 2014, from 11.9 to 63.3 per 100,000 male population and from 3.7 to 13.8 per 100,000 female population, with a corresponding increase in mortality rate (9.1 to 32.4 in men and 2.3 to 12.4 in women) (22). In 2020, the International Agency for Research on Cancer (IARC) reported that lung cancer ranked fifth among all cancers, accounting for 4.9% of all new cancer cases in Egypt, with a 5-year prevalence of 6.95 per 100,000 population and a mortality rate of 6.5% (23). Similarly, according to the IARC2020 data from Jordan, lung cancer ranked second among all cancers in both sexes with an incidence of 9.1%, a 5-year prevalence of 10.85, and a mortality rate of 15.2% (24).
Conforming to global trends, lung cancer prevalence in the MEA region shows a strong male preponderance. However, an increase in lung cancer among females in the region has been observed, probably echoing the upsurge of smoking in females in recent years (10,25). Up to 2015, lung cancer showed a significantly increasing trend for both men and women in Lebanon, having the second highest incidence among men and highest incidence among women compared with other MEA countries (21). In UAE, the reported incidence of lung cancer was 7.8% of 425 total cases in men and 1.0% of 680 total cases in women in 2017, with an estimated average mortality of 8.4% (20). Qatar has also reported a rise in the lung cancer incidence among women, while the male-female ratio of lung cancer incidence in Egypt from 1980 to 2014 was reported to be 3.2:1 (22,25).
The age-standardized incidence rate (ASIR), as well as the age-standardized mortality rate (ASMR) of lung cancer in the MEA region (ASIR, 17.5 per 100,000 people; ASMR, 15.7 per 100,000 people), were found to be less than the global rates (ASIR, 22.4 per 100,000 people; ASMR, 18.0 per 100,000 people). Turkey had the highest ASIR and ASMR per 100,000 people for lung cancer among the MEA countries which were both significantly higher than the global average (ASIR: global, 22.4; Turkey, 40.0; ASMR: global, 18.0; Turkey, 35.9). The lowest ASIR (2.5) and ASMR (2.3) among the MEA countries was found in Sudan, which were significantly lower than the average for the region as well as the global average (Table 1) (2,13,26).
Table 1
| Countries | ASIR/ 100,000 people |
ASMR/ 100,000 people |
|---|---|---|
| Algeria | 11.5 | 10.4 |
| Armenia | 27.8 | 25.5 |
| Bahrain | 11.7 | 10.6 |
| Egypt | 8.0 | 7.2 |
| Iraq | 12.0 | 11.1 |
| Jordan | 15.6 | 14.3 |
| Kuwait | 8.0 | 7.2 |
| Lebanon | 18.7 | 16.6 |
| Libya | 16.6 | 14.9 |
| Morocco | 18.3 | 16.3 |
| Oman | 5.4 | 5.0 |
| Qatar | 9.2 | 8.5 |
| Saudi Arabia | 5.1 | 4.6 |
| South Africa | 18.3 | 15.8 |
| Sudan | 2.5 | 2.3 |
| Syrian Arab Republic | 15.3 | 14.1 |
| Tunisia | 19.5 | 17.4 |
| Turkey | 40.0 | 35.9 |
| United Arab Emirates | 6.6 | 5.7 |
| Yemen | 5.8 | 5.5 |
ASIR, age-standardized incidence rate; ASMR, age-standardized mortality rate.
Risk factors
Lung cancer is linked to a constellation of well-established risk factors ranging from lifestyle to environmental and occupational exposures to carcinogens (8,13).
Modifiable risk factors
Smoking
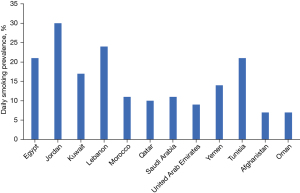
Tobacco smoking has the strongest association of all risk factors in the pathogenesis of lung cancer. The combustion of tobacco emits about multiple carcinogens including polycyclic aromatic hydrocarbons and nitrosamines (27,28). The risk of developing lung cancer is 20 to 50 times higher in current smokers than neversmokers (20). Smokers are also at 20 times higher risk of dying from lung cancer than non-smokers (14). Although cigarette smoking is more common, people in the MEA region also use other kinds of tobacco-based smoking products such as cigarillos, bidi, cigars, pipes, midwakh (a smoking pipe of Arabian origin that is mainly produced in Iran and UAE and made by mixing dokha [a sifted tobacco product of Iranian origin] with aromatic leaves and barks of herbs), shisha (a mixture of tobacco and fruit or molasses sugar), and hookah or waterpipes (10,29). These products are said to pose an equivalent if not higher risk of developing lung cancer. For example, smoking one shisha pipe is equivalent to smoking 100 cigarettes in a day while hookah or waterpipes have high benzene and other carcinogenic content (10,29). Despite having tobacco control policies in place in many MEA and Eastern Mediterranean countries, smoking is highly prevalent in the region, with current data showing approximately 42% of the population smoking some form of tobacco, and this figure is expected to reach 62% by 2025 (13). In a retrospective cohort study conducted in Qatar between 2011 and 2018, approximately 66.4% of the total reported lung cancer cases were found to have a smoking history (25). The recent World Health Organization (WHO) report on the global tobacco epidemic 2021 indicated that the daily smoking prevalence in adults in the MEA region was highest in Jordan (30%) followed by Lebanon (24%), and lowest in Oman (7%) and Afghanistan (7%) (Figure 1), with the age-standardized prevalence being 35.0% for both Jordan and Lebanon, and 8.1% for Oman (30,31). In Turkey, the ban on indoor public smoking was extended to include bars, restaurants, coffee houses, and hookah bars on 19 July 2009. Any form of advertising on smoking was forbidden including depiction of people smoking on television, such as cigarettes blurred out in smoking scenes. The law was upgraded to require plain packaging of all tobacco products with health warnings messages and images covering both sides of packages in at least 85% of the packaging by December 2018. While these efforts were aimed to decrease the number of new smokers, the already existing high number of smokers in the population were at a high risk of developing lung cancer.
Adult men and women of all age groups in the MEA region are habituated to smoking, including physicians (30.7%), university students (19.2%), medical students (25.8%), and pregnant women (20%) (32,33). While a systematic review conducted by Nasser et al. from 2018 to 2019 found higher smoking prevalence in men compared to women in Yemen, Tunisia, Bahrain, Egypt, KSA, Jordan, Palestine, and Syria, another study claimed a higher smoking prevalence in women (15.7%) than men (10.3%) in Yemen (34). A study conducted by Sibai et al. on the Lebanese population found smoking to be common among the younger adults (44.6%) between 18 to 34 years of age affecting a higher percentage of women (53%) than men (47.0%) (32). The WHO data on smoking showed a higher smoking prevalence in Egypt, Jordan, Tunisia, and UAE in men while in Lebanon smoking was higher in women (10,20). Several countries in the MEA region have also reported a surge of smoking among adolescents (13,21). A study evaluating cigarette and waterpipe smoking from 2018 to 2019 among university students found a higher overall smoking rate in Egypt (46.7%), Kuwait (46%), and KSA (42.3%) with approximately 10% prevalence among girls aged 13 to 15 years in 12 Arab countries (34). The Global Youth Tobacco Survey reported smoking prevalence among boys and girls aged 13 to 15 years in the MEA region was 19.8% and 10.6%, respectively (13).
Not just active smoking but passive smoking or exposure to second-hand smoke may equally contribute to the development of lung cancer (27). Non-smokers are at 20% to 30% risk of developing lung cancer when married to a smoker (20). Factors such as duration and frequency of smoking contribute to increasing the vulnerability to lung cancer (27). Occasional smokers and individuals smoking a few cigarettes in a day are also at higher risk of developing lung cancer. The risk goes on, increasing with increasing years of smoking and the number of cigarettes smoked in a day (35). Khattab et al. reported a high cumulative cigarette smoke exposure in both men (77.5%) and women (59.3%) smoking approximately 10 pack-years. The mean cumulative exposure was found to be 29.1±26.2 pack-years for men and 18.5±20.5 pack-years for women. Lebanon and Jordan were reported to have the highest cumulative exposure to cigarette smoke (36). Although, Egypt is reported to consume approximately 20 billion cigarettes annually, the use of waterpipes and imported tobacco are also common. In the Mediterranean region, Morocco is believed to be one of the highest tobacco consumers with more than 15 billion cigarettes smoked every year (13). However, it has been observed that while men and women are equally vulnerable to the development of lung cancer, irrespective of smoking status, there was a higher prevalence of lung cancer among non-smoking women compared to non-smoking men (37).
Other risk factors
Other contributing factors that either individually or in conjunction with smoking increase the risk of lung cancer in an individual are indoor pollution due to unprocessed biomass fuels, air pollution, exposure to radiation and gases like radon, and occupational hazards such as exposure to carcinogenic agents like asbestos, nickel, arsenic, and chromium (27). Burning incense (bakhour) is a popular traditional practice in the MEA region which may also contribute to the risk of lung cancer (38-40). Lebanon has relatively high air pollution due to the excessive use of diesel-operated electric generators. Diesel engine exhaust is known to increase the relative risk of lung cancer by 30% to 50% (21). Presence of other concomitant respiratory problems like asthma and chronic obstructive pulmonary disorder also has a significant role in the development of lung cancer (21). Human immunodeficiency virus (HIV) infection, which is highly prevalent in many parts of Africa, has also been shown to independently increase the risk of lung cancer (41). Evidence also suggests that lung cancer in people living with HIV presents at a younger age with a significantly worse performance status and almost exclusively with incurable disease at presentation (41,42).
Non-modifiable risk factors
Age is a key determinant of the risk of the development of lung cancer. Individuals in the advanced age group (55 to 74 years) are more susceptible to developing lung cancer than the younger population (37). An epidemiological study by Jaafar et al. conducted in four Gulf countries found that more than 50% of the included patients with lung cancer were >45 years of age (43). Another study conducted in Qatar between 2011 and 2018 found that the majority of patients with lung cancer (56.1%) were in the age group of 50 to 69 years (25). In Yemen and Qatar, the median age of lung cancer detection is reported to be 60 years (13,25,43). In Egypt, mean age of lung cancer diagnosis was found to be 48 years, with the majority of patients being in the age range of 50 (55.3%) to 60 (29.3%) years at diagnosis (22). Salhab et al. reported that the incidence rate of lung cancer increased with age in both Lebanese men and women and 89.2% of the cases were ≥50 years of age (21).
A positive family history of lung cancer increases the vulnerability of developing lung cancer in an individual (8). Certain population groups tend to have a predisposition to developing lung cancer over others. For example, African American individuals from a low socioeconomic background with a low body mass index, a personal history of cancer, and a family history of lung cancer are at a greater risk of developing lung cancer, even if not exposed to any other risk factor (44). Similarly, East Asians seem to have an inherent non‑environmental susceptibility to the development of lung cancer associated with mutations in the epidermal growth factor receptor (EGFR) gene (45). Genetic mutations such as EGFR, anaplastic lymphoma kinase (ALK), c-ros oncogene 1, Kirsten rat sarcoma virus (KRAS), V-raf murine sarcoma oncogene homolog B1 (BRAF), mesenchymal-epithelial transition factor (MET), human epidermal growth factor receptor 2 (HER2), and other genes are common driver mutations of advanced NSCLC (Table S2) (11,45,46). The epidemiological patterns of these genetic alterations vary according to geographical areas, ethnicity, sex, and pathological factors. Molecular therapies are currently targeting these genetic driver mutations of lung cancer.
Screening criteria for lung cancer
Current guidelines recommend that lung cancer screening should target individuals considered to be at the highest risk, who are also most likely to benefit from the screening. As tobacco smoking is the primary risk factor for lung cancer, screening criteria customarily focus on smoking status for selecting high-risk individuals (44,47). However, smoking status is insufficient to identify all individuals at high risk of lung cancer. Hence, risk prediction models are incorporating important risk factors for lung cancer such as a family history of cancer or respiratory illness, occupational exposures, race, and ethnicity are helpful tools for identifying individuals at high-risk who might be missed if only their age and smoking status were considered (48,49). Patients with underlying conditions such as emphysema, pulmonary fibrosis, positive family history, and known exposure to inhaled carcinogens are included in the high-risk group. Most countries in the MEA region do not have any set lung cancer screening programs. However, in the majority of countries within the region, the criteria for identifying high-risk populations who are eligible for screening should meet the following (20,50,51):
- Age 50 to 80 years in general good health and fit for further investigations;
- Individuals who smoked or had at least a 20-pack-year history of smoking;
- No prior history of lung cancer (in this case follow-up and not screening is required);
- A family history of lung cancer.
Guidelines for early detection of lung cancer
Many lung cancer screening trials with chest radiography and LDCT have been conducted over the past 40 years among heavy smokers (52). The Early Lung Cancer Action Project revealed that LDCT was more sensitive than chest radiography in lung cancer detection (52). Several other studies conducted later showed that LDCT detected more nodules during early-stage disease than chest radiography (20). The landmark National Lung Screening Trial (NLST) compared the efficacy of two imaging techniques (LDCT and chest radiography) to determine the efficacy of LDCT screening in reducing mortality from lung cancer. The study results showed a 20% [95% confidence interval (CI): 6.8% to 26.7%; P=0.004] reduction in mortality from lung cancer with LDCT screening (18,19,29,47,53). The enrolment criteria of NLST trial majorly focus on age and smoking history in order to identify high-risk individuals for screening thereby missing the inclusion of a substantial number of people with probable risk of developing lung cancer (20,50). Following NLST, another randomized controlled trial, the Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON) was undertaken on individuals who smoked or had a history of smoking between 50 and 75 years of age and a history of smoking >15 cigarettes daily for >25 years or >10 cigarettes daily for >30 years and ≤10 years after quitting smoking (20,50). The results showed that more than half of the screening-detected cases were in the early stages (IA and IB) (20,50). The NLST and NELSON trials were conducted to determine the effectiveness of volume based LDCT screening in reducing lung cancer-associated mortality. The findings revealed screening using LDCT detected almost 2/3rd of the cases in the early stages and helped in reducing lung cancer-related mortality by almost 24% in men and 33% in women (20). The NLST and NELSON selection criteria are now widely used for identifying eligible candidates for lung cancer screening (18,19,29,47,53). In another trial, the International Early Lung Cancer Action Project (I-ELCAP), screening resulted in a diagnosis of early-stage lung cancer in 85% of the participants and the estimated 10-year survival rate was 88% in this subgroup (95% CI: 84% to 91%) (54). Based on the findings of the NLST, NELSON trial, and other key trials (Table S3), the American Cancer Society, the National Comprehensive Cancer Network (NCCN), as well as the National Health Service of the United Kingdom released lung cancer screening guidelines targeting individuals aged 55 to 74 years who smoked or had a history of smoking 30 pack-years (53,55,56). In March 2021, the United States Preventive Services Task Force published substantial revisions in lung cancer screening recommendations that suggest that screening for lung cancer with LDCT is recommended in adults aged 50 to 80 years who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years (57).
Lung cancer risk prediction models
Risk-prediction models that take into consideration pulmonary nodule size, calcification, spiculation, density, and other imaging results are recommended by several clinical practice guidelines for the selection of patients for LDCT based on high-risk criteria (58-60). These models aim to stratify risk of malignancy in nodules detected on computerized tomography (CT) and are effective in distinguishing benign and malignant cases (61,62). The risk prediction models are frequently categorized into traditional models that take into account raw data and deep learning algorithm-based models, wherein the use of raw data is allowed with representations needed for detection or classification.
These risk prediction models have been validated and found effective as well as cost-effective. There are currently more than 18 traditional models developed to predict the pathological characteristics of pulmonary nodules. Of these models, 7 were based on the North American population, 2 were based on the European population, and 9 were based on the Asian population (63). Further, various predictive models have also been developed that estimate the overall risk of developing lung cancer based on various risk factors, including demographic, clinical, and lifestyle factors. These models are valuable for identifying high-risk individuals who may benefit from preventive measures, screening programs, or closer monitoring. A comparative analysis of highrisk screening using a risk prediction model versus standard care using data from the NLST reports that the use of the risk prediction tool would have reduced the number of individuals requiring screening in the NLST by 81% (64). Keeping in mind the limitations of the NLST trial, another risk-prediction model with high predictive accuracy called Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial 2012 Model Version 2012 (PLCOm2012) was developed. The model aids in stratifying the patients being screened into high- and low-risk groups and in determining the screening interval (44). It takes into consideration risk factors such as age, race, body mass index, chronic obstructive pulmonary edema, education, a family history of lung cancer, a personal history of cancer, smoking status, and duration since smoking cessation in the case of individuals with a history of smoking. The 6-year risk score cut-off of 1.5% is the eligibility criteria for the screening with LDCT with biennial screening programs reported to be highly cost-effective (44,47).
Screening guidelines for pulmonary nodule management in lung cancer patients
Several international guidelines including the Fleischner Society (65), British Thoracic Society (59,66), American College of Clinical Pharmacy (67), NCCN (68), the Asian guidelines (69), as well as the Saudi Lung Cancer Screening guidelines (70) and the South African Thoracic Society guidelines (50), primarily recommend LDCT for pulmonary nodule assessment and provide recommendations and algorithms for appropriate follow-up and management of lung nodules. LDCT screening is helpful in detecting a significant number of patients with lung cancers at a very early stage in the never-smoker population also (71). Older age (≥50 years) and heavy smoking (≥20 pack-years) are identified as risk factors by most of the guidelines while high-risk nodule features include large size, irregular or spiculated margins, and upper lobe location. Solitary solid nodules <6 mm do not require routine follow-up in patients at low risk of cancer. High-risk patients with solitary solid nodules <6 mm are recommended for annual screening with LDCT until they are no longer candidates for definitive treatment (65,68). For a solitary solid nodule in patients with high risk of cancer measuring 6 to 8 mm, a follow-up CT at 6 to 12 months then 18 to 24 months is recommended. For high-risk solid nodules measuring >8 mm, a follow-up CT or tissue-sampling is recommended at 3 months. In case the doubling time for the size of the nodules is between 100 to 400 days or if there is clear evidence of nodule growth, a positron emission tomography (PET)/CT or tissue sampling is recommended (65,68). A part-solid nodule is suggestive of inflammatory causes or peripheral adenocarcinoma with follow-up duration depending on the growth of the solid part of the nodule. Solitary part-solid nodules measuring ≥6 mm are followed-up at by CT at 3 to 6 months to confirm persistence. If unchanged and the solid component remains at the same size, annual CT is recommended for 5 years or until the patient is no longer a candidate for definitive treatment (65,68). Hence, effective screening requires a personalized approach based on individual patient characteristics and the characteristics of the pulmonary nodules. Regular follow-up and collaboration among healthcare providers are crucial for achieving optimum patient outcomes.
Screening practice for lung cancer across MEA region
Screening practices for lung cancer across MEA countries are marked by significant variations in implementation and utilization. Although certain countries have the established guidelines and resources, the majority of the other MEA countries are yet to implement a structured lung cancer screening program. However, the findings from the existing screening programs in some MEA countries should guide lung cancer screening programs in others (Table 2). Lung cancer policy network provides a platform to showcase results of ongoing and completed research on LCDT screening globally. The map facilitates sharing of information on best practices on use of LDCT, to guide development of policy regarding the use of LDCT screening in lung cancer diagnosis. The information obtained from this interactive map can be used by policymakers to implement policy changes on lung cancer detection (72). It is crucial to acknowledge that effectively addressing the diverse challenges and opportunities in MEA countries demands a considerate and adaptable approach tailored to each nation’s unique characteristics. Collaboration among governments, international organizations, and the private sector remains pivotal for the successful implementation of robust and equitable lung cancer screening programs across the region.
Table 2
| Countries | Key points |
|---|---|
| UAE (20,29) | Risk factors for lung cancer: |
| ❖ Age: between 55 and 74 years | |
| ❖ Family history of lung cancer | |
| ❖ Passive or active smoker (current or former heavy smokers: at least one cigarette pack daily for 30 years or two packs for 15 years) | |
| ❖ Air pollution | |
| ❖ Exposure to asbestos, radon, and diesel exhaust | |
| Indications for urgent chest X-ray (to be performed within 2 weeks to access for lung cancer): | |
| ❖ People aged ≥40 years and experiencing ≥2 of the following unexplained symptoms, or if current or former smoker having ≥1 of the following unexplained symptoms: | |
| • Cough | |
| • Fatigue | |
| • Shortness of breath | |
| • Chest pain | |
| • Weight loss | |
| • Appetite loss | |
| ❖ People aged ≥40 years with any of the conditions: | |
| • Persistent or recurrent chest infection | |
| • Finger clubbing | |
| • Supraclavicular lymphadenopathy or persistent cervical lymphadenopathy | |
| • Chest signs consistent with lung cancer | |
| • Thrombocytosis | |
| Indications for LDCT: | |
| ❖ Age: between 55 and 74 years | |
| ❖ Family history of lung cancer | |
| ❖ Passive or active smoker (current or former heavy smokers: at least one cigarette pack daily for 30 years or two packs for 15 years) and recommended for annual screening with LDCT | |
| ❖ A PLCOm2012 6-year risk score of >1.51% (47,73) | |
| ❖ With the following suspicious chest X-ray findings: | |
| • A nodule or mass | |
| • Multiple pulmonary nodules | |
| • Non-resolving pleural effusion | |
| • Mediastinal or contralateral hilar adenopathy | |
| • Interstitial infiltrates | |
| • Slowly or non-resolving pneumonia or consolidation | |
| • Apical fibrotic disease suggestive of possible tuberculosis | |
| • Unexplained elevated diaphragm | |
| ❖ Age ≥40 years with unexplained hemoptysis | |
| ❖ Person with hemoptysis and related symptoms despite normal chest X-ray findings | |
| Saudi Arabia (70) | Eligible candidates for screening are as follows: |
| ❖ Asymptomatic patients (for screening symptomatic patients, standard procedure of care to be followed) | |
| ❖ Age 55 to 77 years | |
| ❖ Smoking history of ≥30 pack-years | |
| ❖ Active smoker or having quit smoking <15 years ago | |
| ❖ No chest CT scan done in the last year | |
| Non-eligible candidates/conditions: | |
| ❖ Individuals with comorbidities that could adversely influence their ability to tolerate the evaluation of screen-detected findings or tolerate treatment of early-stage screen-detected lung cancer or that substantially limit their life expectancy | |
| ❖ LDCT screening not to be performed in case of: | |
| • Advanced liver disease | |
| • COPD with hypoventilation and hypoxia | |
| • NYHA class IV heart failure | |
| Recommendations post-CT scan findings during screening: | |
| ❖ Normal CT scan to be performed after 1 year and then repeated annually | |
| ❖ Positive findings to be compared with previous imaging data if available | |
| ❖ New lung lesions: | |
| • Size <5 mm: repeat CT in 12 months and thereafter depending on CT results | |
| • Size 6 to 7 mm: repeat CT in 6 months | |
| • Size >8 mm: refer to pulmonary/thoracic surgeon for workup | |
| South Africa (50) | Eligibility criteria for annual screening with LDCT: |
| ❖ Age 55 to 74 years | |
| ❖ Current or former smokers (having quit within the preceding 15 years) with at least a 30-pack-year history | |
| ❖ No history of lung cancer | |
| ❖ Are in general good health and fit for surgery | |
| Recommendations for LDCT screening: | |
| ❖ Screen annually until 15 years have passed from date of smoking cessation, or until 80 years of age, become unfit for a curative surgery or until significant changes are observed | |
| ❖ Nodule size ≥6 mm is considered threshold for positive lesion at baseline | |
| ❖ Upon LDCT findings: | |
| • Solid or partly solid nodule is ≥6 mm, but <10 mm with no malignant features: LDCT to be repeated every 6 months | |
| • Solid nodule or the largest solid component of a non-solid nodule is ≥10 or ≥6 mm and enlarging or with additional malignant features present. Considered as positive nodules and recommended for definitive diagnosis and/or therapeutic intervention | |
| Recommendations for LDCT protocols within a TB-endemic setting: | |
| ❖ Tuberculosis is highly prevalent in South Africa, which masks symptoms of lung cancer. Thus, a clearly documented protocol is developed that is specific to each LDCT scanner |
UAE, United Arab Emirates; LDCT, low-dose computerized tomography; CT, computerized tomography; COPD, chronic obstructive pulmonary edema; NYHA, New York Heart Association; PLCOm2012, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial 2012 Model Version 2012; TB, tuberculosis.
Factors leading to delay in lung cancer diagnosis across MEA region
Early detection rate is still low in the MEA region, with many countries including UAE reporting only one-fourth of the total cases as being detected at early stages (stage I and II) and majority detected at advanced stages (20). Factors responsible for delayed diagnosis of lung cancer vary between countries (10,14,74,75). The majority of the countries lack an early screening and patient referral program for lung cancer (20,29). While regular screening may be practiced in high-risk individuals independently by a few physicians, the region lacks a mass screening program using LDCT at the national level. LDCT is associated with certain risks such as excessive procedures, follow-ups, cumulative exposure to radiation during scheduled yearly screening, false-positive findings, and hence anxiety among the patients. Owing to benign and noncancerous findings during LDCT screening, the cost-effectiveness of the screening procedure is still under question in the region (20,76,77). Additionally, inconsistencies in levels of awareness about screening guidelines and eligibility, and lack of understanding of the risks and benefits of screening among healthcare professionals in the primary care setting have been evidenced in some countries often resulting in misinterpretation of imaging reports (10,13,14,29,76). Overlapping symptoms with other respiratory diseases such as tuberculosis often delay diagnosis thereby delaying referral to the oncologists and resulting in patients getting incorrect treatment for extended periods (29,75). Lack of a documented patient referral pathway in several countries of the MEA often results in inadequate communication between primary care physicians (PCPs) and oncologists (10). Some of the low-middle-income MEA countries including Lebanon have limited healthcare funding. This not only causes delayed lung cancer detection but also results in an underestimation of the lung cancer prevalence rate (21). Additionally, the absence of population-based registries for high-risk individuals and automation in diagnostic tools may delay the early identification of patients with lung cancer. Large-scale screening programs may help to catalyze the development of supporting networks and capacity. The most common reasons for the delayed diagnosis of lung cancer in the MEA region include the following:
- Lack of awareness among individuals about disease symptomatology, failure to act after experiencing symptoms due to negligent behavior or fear;
- Lack of awareness among the general public about smoking as a major causative factor;
- Lack of exploration of the risk-benefit ratio of LDCT due to the misconception of perceived risks associated with the procedure resulting in lower compliance for screening;
- Lack of a reliable database of the entire population that includes smoking history and other relevant risk factors to determine screening eligibility;
- Low or non-adherence to screening guidelines by many physicians;
- Lack of expertise among PCPs in early lung cancer diagnosis due to inadequate training on disease symptomatology and progression;
- Lack of adequate referral pathways;
- Early diagnosis may be missed due to inability to make a differential diagnosis in presence of comorbid conditions with overlapping symptoms with other respiratory diseases like tuberculosis and pneumonia;
- Lack of a standard procedure or early patient referral pathways in the region;
- Poor access to many diagnostic options or tests such as tumor molecular profiling in several countries in the region. Precession diagnostic modalities are available only in limited countries and tertiary centers;
- Facilities for diagnosing EGFR and ALK receptor tyrosine kinase mutations, or imaging modalities such as PET/CT are available in some countries only.
Strategies for effective screening and referral for early diagnosis of lung cancer
In the MEA region, most patients with lung cancer have a poor prognosis at presentation since most of the cases are diagnosed in advanced stages hence, limiting the availability of treatment choices (3,10,13). According to Cancer Research United Kingdom, the majority of patients with lung cancer are likely to survive for at least a year if diagnosed at an early stage compared with approximately 15% of patients diagnosed at an advanced stage of the disease (2,20). Hence, to optimize lung cancer management and to reduce lung cancer burden in the MEA region, it is imperative to adopt screening programs for highrisk individuals and have a well-documented referral pathway to be followed as part of clinical practice across the region (2,20). Recommendations for effective screening and patient referral for lung cancer include:
- Educating the general public about disease symptomatology, associated risk factors (especially smoking), and benefits of early screening where appropriate;
- Initiating awareness programs about lung cancer and smoking targeted towards different ethnic groups with major focus on adolescents and those from low socioeconomic status;
- Establishing well documented criteria for identifying high-risk patients;
- Formulating a well-documented patient referral pathway;
- Risk-prediction models may be used to predict the risk for malignancy before referral for LDCT.
- Arranging training programs for PCPs to guide them referral pathway for screening;
- Establishing cancer registries in the region to have a reliable and updated lung cancer epidemiological data, to aid in strategic planning for prevention, control, and treatment of lung cancer.
The quality-of-care pathways determine the success of a screening programs. Every suspected case of lung cancer should have immediate access to accurate diagnosis and care led by a multidisciplinary team (MDT) (78). Patient barriers to attending the screening may be physical, financial, informational, social, or cultural. Compliance to the screening is one of the key milestones to be achieved to improve patient outcomes (20,21). As PCPs are the frontline providers in the healthcare systems, effective screening strategies and training programs for PCPs will guide them in issuing referrals for high-risk patients for early screening (20,74). Furthermore, it is essential to have programs focusing on developing a cancer registry in the region in order to have comprehensive, reliable, and updated lung cancer epidemiological information and to aid strategic planning for the prevention, control, and treatment of lung cancer (13,22,25). Also, encouraging more genetic and epigenetic studies will help better evaluate the effect of genetic factors in the development of lung cancer (21).
Recommended referral programs for improving early lung cancer detection
Due to uncertainties regarding the yield and cost-effectiveness of LDCT in MEA countries, physicians often adopt an individualized approach to screen high-risk patients, utilizing either chest radiographs or LDCT (12). However, compliance with guidelines for screening and early referral to a specialist and accurate prompt treatment are greatly improve patient outcomes. Since high-risk individuals are initially treated by PCPs, experts have proposed a pathway to help PCPs prescribe early screening imaging referrals based on the NLST, NELSON, and PLCOm2012 criteria. Figure 2 illustrates the general sequence of referrals following a chest radiography (53,55).

A well-documented referral guideline for PCPs in the MEA region is urgently required focusing on risk factors and patients’ criteria for referral. For patients with specific symptoms suggestive of lung cancer like hemoptysis, guidelines suggest that PCPs need to urgently refer for imaging as well as refer to a specialist linked to a lung cancer MDT (20). The effectiveness of the referral program may be monitored through key performance indicators like the time from the abnormal radiological findings to the treatment initiation. An MDT comprising pulmonary specialists, radiologists, pathologists, oncologists, and thoracic surgeons may be developed to collaborate for diagnosis post-LDCT and minimize false-positive results, unnecessary invasive procedures, and design diagnostic pathways.
Emerging role of AI for lung cancer screening
Emergence of AI based screening techniques have offered advancement in lung cancer management and is gaining popularity worldwide. The use of such technology not only helps in reducing a radiologist’s burden but also increase the sensitivity and specificity of detection. The use of AI in the evaluation of pulmonary images can greatly improve the accuracy of identifying pulmonary nodules in primary care settings, leading to earlier diagnosis. The AI uses an algorithm that supports radiologists in interpreting radiological reports accurately, thus helping in faster and more accurate diagnosis (79,80). Computer-aided diagnosis (CADx) schemes improve radiologists’ diagnosis accuracy while shortening the time to view CT images (81). CADx and computer-aided detection (CADe) are two elements of the CAD system for nodule detection. The CADe attempts to segregate nodule and no nodule candidates (anatomical structures such as blood vessels and tissues), while the CADx characterizes and classifies the segmented lesions as a cancerous tumor or noncancerous tumor. Results from a study conducted by Mahboub et al. in the UAE supported the use of a machine-learning model as a passive tool for monitoring all X-rays processed at an institution to find incidental cases of lung cancer or as a triaging tool for facilitating the patients’ journey through the standard care pipeline for lung cancer (82). However, as most of the countries in the MEA region including Lebanon, Egypt, Turkey, and KSA have limited real-time experience in use of AI-based algorithms in lung cancer screening, there is a need for creating awareness about the latest technologies among physicians and other stakeholders and provide training to the healthcare providers to encourage use of AI aided screening and diagnostic techniques in the clinical practice in the region.
Conclusions
The incidence of lung cancer has increased significantly in the MEA in region recent years. Lack of awareness about disease symptoms, misdiagnosis, limited screening initiatives, and late referral to specialists are the major reasons for delayed diagnosis. The high incidence and poor survival rates of patients with lung cancer in the region are primarily due to delayed presentation and diagnosis, emphasizing the need for national-level lung cancer screening programs. High cost, lack of public awareness, and lack of expertise among the healthcare providers prevent the judicious use of LDCT in the MEA region. A well-established screening and referral guideline can aid the healthcare providers in early identification enabling timely intervention and reducing delays. Research on risk factors, awareness programs for physicians and patients and integration of lung cancer screening initiatives with tobacco control programs have the potential to improve clinical outcomes by facilitating higher adherence to screening and may also prove to be cost-effective over the long term. This holistic and evidence-based approach also supports rational decision-making for fund allocation, emphasizing high-impact interventions to reduce the overall burden of lung cancer. Local governments in the MEA region need to be made aware of the importance of lung cancer screening and should be convinced to initiate large-scale programs targeting high-risk individuals to improve survival outcomes.
Acknowledgments
The authors would like to thank Dr. Debasri Mukherjee and Dr. Chinmayee Joshi of Fortrea Scientific Services Pvt Ltd (formerly Labcorp Scientific Services & Solutions Pvt. Ltd.) for medical writing support in accordance with GPP 2022 guidelines.
Funding: The preparation of this expert consensus manuscript and funding of the journal’s article processing charges was supported by
Footnote
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1568/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1568/coif). All authors report that the preparation of this expert consensus and funding of the journal’s article processing charges were supported by AstraZeneca FZ LLC. H.O.A.S. received research funding from Roche Pharmaceutical and Merck Pharmaceutical. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO. World health statistics 2022 – Monitoring health for the SDGs [Internet]. 2022. Available online: https://www.who.int/data/gho/publications/world-health-statistics
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- The Lung Ambition Alliance. Lung Cancer Screening: The Cost of Inaction [Internet]. IASCL; 2021 [cited 2022 Nov 17]. Available online: https://www.lungambitionalliance.com/our-initiatives/lung-cancer-screening-the-cost-of-inaction.html
- International Agency for Research on Cancer. World Health Organization. Lung Factsheet: Globocan; 2020. [Internet]. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf
- Tindle HA, Stevenson Duncan M, Greevy RA, et al. Lifetime Smoking History and Risk of Lung Cancer: Results From the Framingham Heart Study. J Natl Cancer Inst Monogr 2018;110:1201-7. [Crossref] [PubMed]
- Cufari ME, Proli C, De Sousa P, et al. Increasing frequency of non-smoking lung cancer: Presentation of patients with early disease to a tertiary institution in the UK. Eur J Cancer 2017;84:55-9. [Crossref] [PubMed]
- Siegel DA, Fedewa SA, Henley SJ, et al. Proportion of Never Smokers Among Men and Women With Lung Cancer in 7 US States. JAMA Oncol 2021;7:302-4. [Crossref] [PubMed]
- Barta JA, Powell CA, Wisnivesky JP. Global Epidemiology of Lung Cancer. Ann Glob Health 2019;85:8. [Crossref] [PubMed]
- Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res 2015;4:327-38. [Crossref] [PubMed]
- Al-Shamsi HO, Abu-Gheida IH, Iqbal F, et al. editors. Cancer in the Arab World [Internet]. Springer Nature; 2022 [cited 2022 Nov 2]. Available online: https://library.oapen.org/handle/20.500.12657/54044
- Jazieh AR, Jaafar H, Jaloudi M, et al. Patterns of epidermal growth factor receptor mutation in non-small-cell lung cancers in the Gulf region. Mol Clin Oncol 2015;3:1371-4. [Crossref] [PubMed]
- Jazieh AR, Bamefleh H, Demirkazik A, et al. Modification and implementation of NCCN guidelines on non-small cell lung cancer in the Middle East and North Africa region. J Natl Compr Canc Netw 2010;8:S16-21. [Crossref] [PubMed]
- Jazieh AR, Algwaiz G, Errihani H, et al. Lung Cancer in the Middle East and North Africa Region. J Thorac Oncol 2019;14:1884-91. [Crossref] [PubMed]
- Salim EI, Jazieh AR, Moore MA. Lung cancer incidence in the arab league countries: risk factors and control. Asian Pac J Cancer Prev 2011;12:17-34.
- Boustany Y, Laraqui A, El Rhaffouli H, et al. Prevalence and Patterns of EGFR Mutations in Non-small Cell Lung Cancer in the Middle East and North Africa. Cancer Control 2022;29:107327482211294.
- Thanh NX, Pham TM, Waye A, et al. Expected Cost Savings From Low-Dose Computed Tomography Scan Screening for Lung Cancer in Alberta, Canada. JTO Clin Res Rep 2022;3:100350. [Crossref] [PubMed]
- Shojaee S, Vachani A, Nana-Sinkam P. The Financial Implications of Lung Cancer Screening: Is It Worth It? J Thorac Oncol 2017;12:1177-9. [Crossref] [PubMed]
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Al-Shamsi HO, Jaffar H, Mahboub B, et al. Early Diagnosis of Lung Cancer in the United Arab Emirates: Challenges and Strategic Recommendations. Clin Pract 2021;11:671-8. [Crossref] [PubMed]
- Salhab HA, Fares MY, Khachfe HH, et al. Epidemiological Study of Lung Cancer Incidence in Lebanon. Medicina (Kaunas) 2019;55:217. [Crossref] [PubMed]
- El-Moselhy EA, Elrifai AW. Risk Factors of Lung Cancer Worldwide and in Egypt: Current Situation. J Oncopathol Clin Res 2018;2:5.
- International Agency for Research on Cancer. World Health Organization. Egypt Lung Factsheet: Globocan; 2020. [Internet]. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/populations/818-egypt-fact-sheets.pdf
- International Agency for Research on Cancer. World Health Organization. Jordan Lung Factsheet: Globocan; 2020 [Internet]. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/populations/400-jordan-fact-sheets.pdf
- Ibrahim WH, Shariff K, El Mistiri M, et al. Featuring Trends in the Epidemiology of Lung Cancer Following the Publication of the National Cancer Strategy in Qatar. Oman Med J 2021;36:e276. [Crossref] [PubMed]
- Estimated age-standardized mortality rates_Globocon_MEA_2020 [Internet]. 2020 [cited 2022 Dec 26]. Available online: https://gco.iarc.fr/today/online-analysis-map?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=15&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&group_cancer=0&include_nmsc=0&include_nmsc_other=0&projection=natural-earth&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=hub_izmir&show_ranking=0&rotate=%255B10%252C0%255D
- Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e1S-e29S.
- Adesina OA, Olowolafe TI, Igbafe A. Levels of polycyclic aromatic hydrocarbon from mainstream smoke of tobacco products and its risks assessment. J Hazard Mater Adv 2022;5:100053.
- Al-Shamsi H, Darr H, Abu-Gheida I, et al. The State of Cancer Care in the United Arab Emirates in 2020: Challenges and Recommendations, A report by the United Arab Emirates Oncology Task Force. Gulf J Oncolog 2020;1:71-87.
- WHO report on the global tobacco epidemic 2021: addressing new and emerging products [Internet]. 2021. Available online: https://www.who.int/publications/i/item/9789240032095
- Abu-Rmeileh NME, Khader YS, Abdul Rahim H, et al. Tobacco control in the Eastern Mediterranean region: implementation progress and persisting challenges. Tob Control 2022;31:150-2. [Crossref] [PubMed]
- Sibai AM, Iskandarani M, Darzi A, et al. Cigarette smoking in a Middle Eastern country and its association with hospitalisation use: a nationwide cross-sectional study. BMJ Open 2016;6:e009881. [Crossref] [PubMed]
- Chidiac A, Tamim H, Kanso M, et al. Smoking among Lebanese medical students: Prevalence and attitudes. Ann Thorac Med 2016;11:183-90. [Crossref] [PubMed]
- Nasser AMA, Geng Y, Al-Wesabi SA. The Prevalence of Smoking (Cigarette and Waterpipe) among University Students in Some Arab Countries: A Systematic Review. Asian Pac J Cancer Prev 2020;21:583-91. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. What Are the Risk Factors for Lung Cancer? [Internet]. 2022. Available online: https://www.cdc.gov/cancer/lung/basic_info/risk_factors.htm#:~:text=Cigarette%20smoking%20is%20the%20number,the%20risk%20for%20lung%20cancer
- Khattab A, Javaid A, Iraqi G, et al. Smoking habits in the Middle East and North Africa: results of the BREATHE study. Respir Med 2012;106:S16-24. [Crossref] [PubMed]
- North CM, Christiani DC. Women and lung cancer: what is new? Semin Thorac Cardiovasc Surg 2013;25:87-94. [Crossref] [PubMed]
- Cohen R, Sexton KG, Yeatts KB. Hazard assessment of United Arab Emirates (UAE) incense smoke. Sci Total Environ 2013;458-460:176-86. [Crossref] [PubMed]
- Dalibalta S, Elsayed Y, Alqtaishat F, et al. A health risk assessment of Arabian incense (Bakhour) smoke in the United Arab Emirates. Sci Total Environ 2015;511:684-91. [Crossref] [PubMed]
- Friborg JT, Yuan JM, Wang R, et al. Incense use and respiratory tract carcinomas: a prospective cohort study. Cancer 2008;113:1676-84. [Crossref] [PubMed]
- Sigel K, Wisnivesky J, Gordon K, et al. HIV as an independent risk factor for incident lung cancer. AIDS 2012;26:1017-25. [Crossref] [PubMed]
- Koegelenberg CF, Van der Made T, Taljaard JJ, et al. The impact of HIV infection on the presentation of lung cancer in South Africa. S Afr Med J 2016;106:666-8. [Crossref] [PubMed]
- Jaafar H, Mohieldin A, Mohsen R, et al. Epidermal growth factor receptor (EGFR) positive non-small-cell lung carcinoma (NSCLC) patients in the Gulf region: current status, challenges, and call for action. J Cancer Prev Curr Res 2020;11:130-4.
- Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med 2013;368:728-36. [Crossref] [PubMed]
- Fois SS, Paliogiannis P, Zinellu A, et al. Molecular Epidemiology of the Main Druggable Genetic Alterations in Non-Small Cell Lung Cancer. Int J Mol Sci 2021;22:612. [Crossref] [PubMed]
- Jazieh AR, Gaafar R, Errihani H, et al. Real-World Data on the Prevalence of Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer in the Middle East and North Africa. JCO Glob Oncol 2021;7:1556-3. [Crossref] [PubMed]
- Tammemägi MC, Ten Haaf K, Toumazis I, et al. Development and Validation of a Multivariable Lung Cancer Risk Prediction Model That Includes Low-Dose Computed Tomography Screening Results: A Secondary Analysis of Data From the National Lung Screening Trial. JAMA Netw Open 2019;2:e190204. [Crossref] [PubMed]
- Kauczor HU, Baird AM, Blum TG, et al. ESR/ERS statement paper on lung cancer screening. Eur Radiol 2020;30:3277-94. [Crossref] [PubMed]
- Ruparel M, Navani N. Fulfilling the Dream. Toward Reducing Inequalities in Lung Cancer Screening. Am J Respir Crit Care Med 2015;192:125-7. [Crossref] [PubMed]
- Koegelenberg CFN, Dorfman S, Schewitz I, et al. Recommendations for lung cancer screening in Southern Africa. J Thorac Dis 2019;11:3696-703. [Crossref] [PubMed]
- Bennji SM, Jayakrishnan B, Al-Kindi AH, et al. Lung cancer screening in the gulf: Rationale and recommendations. Ann Thorac Med 2022;17:189-92. [Crossref] [PubMed]
- Park YS. Lung cancer screening: subsequent evidences of national lung screening trial. Tuberc Respir Dis (Seoul) 2014;77:55-9. [Crossref] [PubMed]
- Wood DE, Kazerooni EA, Baum SL, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:412-41. [Crossref] [PubMed]
- International Early Lung Cancer Action Program Investigators. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. [Crossref] [PubMed]
- Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2019;69:184-210. [Crossref] [PubMed]
- Adult screening programme: Lung cancer [Internet]. Available online: https://view-health-screening-recommendations.service.gov.uk/lung-cancer/
- US Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:962-70. [Crossref] [PubMed]
- Swensen SJ, Silverstein MD, Ilstrup DM, et al. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997;157:849-55.
- Baldwin DR, Callister MEGuideline Development Group. The British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax 2015;70:794-8. [Crossref] [PubMed]
- Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol 2017;18:e754-66. [Crossref] [PubMed]
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. [Crossref] [PubMed]
- Hocking WG, Tammemagi MC, Commins J, et al. Diagnostic evaluation following a positive lung screening chest radiograph in the Prostate, Lung, Colorectal, Ovarian (PLCO) Cancer Screening Trial. Lung Cancer 2013;82:238-44. [Crossref] [PubMed]
- Wu Z, Wang F, Cao W, et al. Lung cancer risk prediction models based on pulmonary nodules: A systematic review. Thorac Cancer 2022;13:664-77. [Crossref] [PubMed]
- Cressman S, Peacock SJ, Tammemägi MC, et al. The Cost-Effectiveness of High-Risk Lung Cancer Screening and Drivers of Program Efficiency. J Thorac Oncol 2017;12:1210-22. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Callister ME, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70:ii1-ii54. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S.
- Wood DE, Kazerooni EA, Aberle D, et al. NCCN Clinical Practice Guidelines in Oncology: Lung Cancer Screening. Version 1.2022. [Accessed June 15, 2022]. Available online: https://www.nccn.org/
- Bai C, Choi CM, Chu CM, et al. Evaluation of Pulmonary Nodules: Clinical Practice Consensus Guidelines for Asia. Chest 2016;150:877-93. [Crossref] [PubMed]
- Jazieh AR, AlGhamdi M, AlGhanem S, et al. Saudi lung cancer prevention and screening guidelines. Ann Thorac Med 2018;13:198-204. [Crossref] [PubMed]
- Kang HR, Cho JY, Lee SH, et al. Role of Low-Dose Computerized Tomography in Lung Cancer Screening among Never-Smokers. J Thorac Oncol 2019;14:436-44. [Crossref] [PubMed]
- Interactive map of lung cancer screening: methodology [Internet]. 2021. Available online: https://www.lungcancerpolicynetwork.com/interactive-map-of-lung-cancer-screening/methodology/
- Lebrett MB, Balata H, Evison M, et al. Analysis of lung cancer risk model (PLCO(M2012) and LLP(v2)) performance in a community-based lung cancer screening programme. Thorax 2020;75:661-8. [Crossref] [PubMed]
- Jazieh AR, Bounedjar A, Al Dayel F, et al. Patterns of diagnostic procedures for lung cancer pathology in the Middle East and North Africa. J Thorac Dis 2019;11:5162-8. [Crossref] [PubMed]
- Ellis PM, Vandermeer R. Delays in the diagnosis of lung cancer. J Thorac Dis 2011;3:183-8. [Crossref] [PubMed]
- Lubuzo B, Ginindza T, Hlongwana K. The barriers to initiating lung cancer care in low-and middle-income countries. Pan Afr Med J 2020;35:38. [Crossref] [PubMed]
- Carter-Harris L, Brandzel S, Wernli KJ, et al. A qualitative study exploring why individuals opt out of lung cancer screening. Fam Pract 2017;34:239-44. [Crossref] [PubMed]
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- Joy Mathew C, David AM, Joy Mathew CM. Artificial intelligence and its future potential in lung cancer screening. EXCLI J 19Doc1552 ISSN 1611-2156 [Internet]. 2020 [cited 2022 Nov 15]; Available online: https://www.excli.de/index.php/excli/article/view/3095
- Goncalves S, Fong PC, Blokhina M. Artificial intelligence for early diagnosis of lung cancer through incidental nodule detection in low- and middle-income countries-acceleration during the COVID-19 pandemic but here to stay. Am J Cancer Res 2022;12:1-16.
- Firmino M, Angelo G, Morais H, et al. Computer-aided detection (CADe) and diagnosis (CADx) system for lung cancer with likelihood of malignancy. Biomed Eng Online 2016;15:2. [Crossref] [PubMed]
- Mahboub B, Tadepalli M, Raj T, et al. Identifying malignant nodules on chest X-rays: A validation study of radiologist versus artificial intelligence diagnostic accuracy. Adv Biomed Health Sci 2022;1:137-43.