Transcatheter aortic valve replacement in the management of aortic insufficiency secondary to left ventricular assist device implantation: a case report
Highlight box
Key findings
• Transcatheter aortic valve replacement (TAVR) could be an effective treatment in our case for restoring ejection fraction in a patient with a left ventricular assist device (LVAD) who developed severe aortic insufficiency (AI).
What is known and what is new?
• Patients with LVAD often develop aortic insufficiency. TAVR has demonstrated advantages over surgical aortic valve replacement in managing LVAD-induced AI, reducing inpatient mortality and improving inpatient outcomes.
• To our knowledge, this is the first report of the use of TAVR to correct new-onset, severe LVAD-induced AI in China.
What is the implication and what should change now?
• Related evidence is too limited to provide commentary on this procedure, and additional investigation is warranted.
Introduction
Left ventricular assist device (LVAD) support is considered either the ultimate therapy for the end-stage heart failure patients or bridging to heart transplantation (1-4). However, the increased incidence of aortic insufficiency (AI) decreases the survival and quality of life in patients with LVAD support. One study reported that after LVAD implantation, 15–52% of patients develop severe AI in one to two years (5). With LVAD support, AI can lead to blood reflux, degraded left ventricular function, and even reduced systemic blood perfusion (6,7), which are associated with decreased 1-year survival in patients with LVAD support (8).
Despite increasing awareness of AI after LVAD implantation, effective strategies for the treatment of this condition have not been well developed. The severity of AI and patients’ clinical status can influence the decisions concerning the operation method. Still, there is a lack of consensus regarding the timing of intervention and the preferred technique for treating AI in this challenging setting.
Based on limited evidence, transcatheter aortic valve replacement (TAVR), although technically challenging, appears to have an advantage over surgical aortic valve replacement (SAVR) in the management of LVAD-induced AI with a reduction in inpatient mortality and better inpatient outcomes, and aortic valve surgery is associated with a higher perioperative morbidity and mortality (9,10). Thus far, however, no report exists concerning TAVR in the treatment of LVAD-induced AI in China. In this paper, we present the first described case of new-onset LVAD-induced AI in China and share our experience on this case. The patient underwent TAVR and achieved significant improvement in functional capacity and symptoms. We present this article in accordance with the CARE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1642/rc).
Case presentation
General information
A 55-year-old male patient was admitted to TEDA International Cardiovascular Hospital on 19 July 2022, due to intermittent chest tightness for one week, which had been aggravated for two days. After admission, the patient developed suddenly worsening symptoms, including persistent dyspnea, sweating, dysphoria, orthopnea, decreased blood pressure, and oxygen saturation.
The patient had a history of operation on 16 July 2021, when LVAD (HeartCon®) implantation and tricuspid valvuloplasty were performed as destination therapy for dilated cardiomyopathy with grade IV cardiac function (as per New York Heart Association) after medical treatment had failed. Trivial aortic regurgitation was present before and after LVAD implantation, and the patient recovered well after the operation and was discharged with regular medicine (Warfarin, Metaprolol, Furosemide) and intermittent clinical visits.
The patient had a history of hypertension for 16 years, which was controlled by oral Enteresto. Blood pressure was in good control. The patient had a history of hyperuricemia for more than one year, which was controlled by oral benzbromarone and sodium bicarbonate. History of other medical conditions, such as diabetes mellitus, hepatitis, and tuberculosis, were denied.
The patient had a smoking history of more than 30 years but had quit smoking three years prior, and a history of alcohol intake of more than 30 years, which he had stopped one year earlier. No family history of cancer was reported.
Examination and diagnosis
Physical examination showed the following: blood pressure, 80/56 mmHg; heart rate, 99 bpm; irregular heart rhythm with mechanical sound of blood pump; and slight edema in the lower limbs.
Laboratory examination indicated that routine blood tests, biochemistry, liver and kidney function, electrolyte levels, myocardial enzyme, prothrombin time, and pro-brain natriuretic peptide were abnormal [red blood cell (RBC) 3.7×1012/L, N-terminal pro brain natriuretic peptide (NT-ProBNP) 16,551 pg/mL, prothrombin time (PT) 28.8s, alanine transaminase (ALT) 67 U/L, aspartic transaminase (AST) 53 U/L, total bilirubin (TBIL) 27.2 µmol/L, direct bilirubin (DBIL) 14.1 µmol/L, creatinine (CREA) 159 µmol/L, uric acid (UA) 928 µmol/L, blood urea nitrogen (BUN) 22.5 mmol/L, lactic dehydrogenase (LDH) 175 U/L].
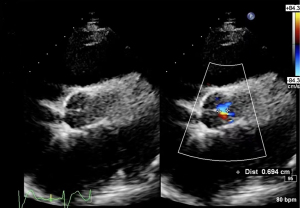
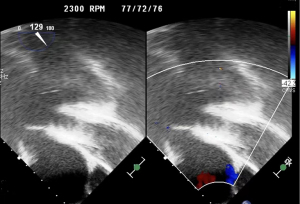
Echocardiography findings included the following: the aortic valve continued to close with obvious reflux signals during closure, left ventricular ejection fraction (LVEF) was 29%, and the artificial pump function was normal (Figures 1,2).
Thoracic computed tomography (CT) post-LVAD implantation indicated that the old lesion was the same as previous, with partial atelectasis of the left lung and trivial effusion in the left thoracic cavity and pericardium.
Coronary artery CT post-LVAD implantation indicated trivial coronary artery calcification without obvious stenosis of the main branch lumen and left-sided heart enlargement with a thinning wall of the left ventricle.
Abdominal aortic CT indicated atherosclerosis of the abdominal aorta and its branches, double iliac atherosclerosis, mild stenosis of the left external iliac artery, and a local penetrating ulcer of the right external iliac artery.
Thoracic aortic CT post-LVAD implantation showed no obvious abnormality.
The overall diagnosis, based on the above-described, was new-onset LVAD-induced AI associated with heart failure.
Treatment
Upon admission, the patient was first treated with tracheal intubation and vasoactive drugs to maintain stable vital signs for heart failure and cardiac arrest. After adjusting the LVAD speed, the patient’s condition was not improved significantly. An expert consultation was conducted with the departments of anesthesiology, radiology, ultrasound, and cardiac surgery, and the intensive care unit (ICU) as well as the others available at our hospital. Given the severity of LVAD-induced AI and sudden cardiogenic shock with unstable hemodynamics, TAVR was performed immediately on the day of admission to correct aortic regurgitation and restore the patient’s hemodynamic stability as soon as possible.
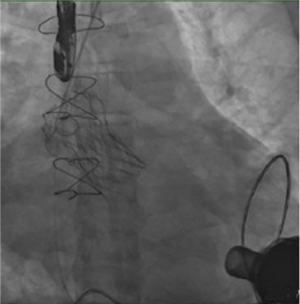
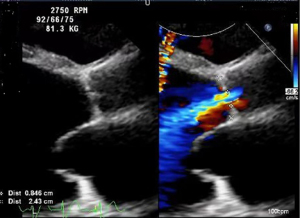
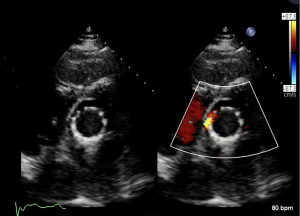
The patient received TAVR under general anesthesia combining intravenous and inhalation anesthesia. A 5F pigtail catheter was positioned at the level over aortic valve through the left radial artery so that aortic root angiography could be performed. Based on the results of the patient’s ultrasound and CT angiography, the average diameter of the aortic sinus was about 30 mm, a 30# self-expandable stent valve (TAV30, Vitaflow Liberty) was selected and positioned at a level slightly over the aortic valve anulus via left femoral artery. Given the expansion of the patient’s valve ring, another 30# valve was positioned again within the prosthetic valve to avoid valve displacement. Subsequently, 26 and 32 mm balloons were used for high-pressure expansion to fix the overlapped valves on the valve ring. The post-deployment image is shown in Figures 2-6. After the LVAD speed was gradually restored, no displacement of transcatheter heart valve prosthesis was observed, and only trivial perivalvular regurgitation was observed, with no regurgitation at the valve orifice, indicating the success of the operation.
Clinical outcomes
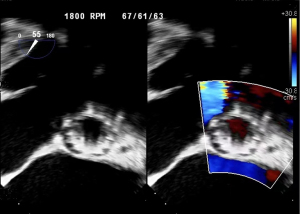
After the operation, the patient demonstrated excellent recovery with the rapid disappearance of chest tightness, stable vital signs, and good performance of the artificial valve. Before discharge, biochemistry, liver function, and electrolytes recovered to normal levels. Echocardiography showed that the position of the artificial valve was fixed. There was no sign of artificial valve insufficiency and no thrombus in the supravalvular and left ventricular outflow tract, yielding an LVEF of 35%. The patient was discharged on 25 August 2022. The patient was hospitalized for 38 days, and followed up with outpatient treatment. On 19 June 2023, the patient’s last follow-up showed that the vital signs were stable, blood tests of hepatic and renal function were normal, LVAD was in working order, with pump speed of 2,600 rpm, pumping flow of 5.27 L/min.
All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Ethical Committee of TEDA International Cardiovascular Hospital [No. (2023)-0310-2]. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
International multidisciplinary team (iMDT) discussion
Discussion among physicians from TEDA International Cardiovascular Hospital
Although a percutaneous intervention strategy without sternotomy to correct severe AI would help avoid surgical risks, the evidence supporting this approach is still limited. To our knowledge, this is the first report on TAVR for correcting new-onset, severe LVAD-induced AI in China.
The morbidity of AI developed by LVAD support seems to rise with the prolonged LVAD support, regardless of the application of autologous valves or prosthetic valves (11,12). Most patients develop severe AI in one to two years after LVAD implantation (5). The progress of AI may be related to the low opening frequency of the aortic valve (8,13-15). With the LVAD support, partial fusion and restriction of the aortic valve leaflets leads to AI (16,17). In our case, the patient displayed such pathologic changes, with continuous aortic valve closure and obvious reflux signals.
Another concern with the development of severe AI with the LVAD support is the preoperative presence of unrecognized, significant AI. Although there is a risk of thrombosis in an autologous aortic valve, the risk of thrombosis is higher in a prosthetic valve replaced after AI (10). Therefore, the treatment principle for the aortic valve is to retain the autologous valve as much as possible, with valvuloplasty being the first choice. If valve replacement is necessary, bioprosthetic valve is recommended due to a high histocompatibility. Based on the current guidelines, an aortic valve intervention performed to correct AI that is greater than mild with the LVAD implantation is the standard clinical strategy and may include valvuloplasty and valve replacement (18). Because the case described in this report did not meet this criterion, aortic valve procedure was not initially performed. It is worth noting that in some studies (19,20), AI before LVAD support was not a significant predictor of AI progression after implantation, with bioprosthetic aortic valve replacement remaining closed during most of the cardiac cycle.
The correction of LVAD-induced AI includes noninvasive and invasive strategies. The primary noninvasive treatment is the controlling of hypertension, including the use of diuretics, vasodilators, and the lowering of the pump speed to reduce left ventricular and LVAD afterload (19,21,22). However, it is difficult to prevent the progression to secondary AI using conventional noninvasive treatment, and patients with severe AI require invasive intervention. In our case, the patient’s condition progressed to severe AI and could not be controlled with conventional noninvasive strategies. Moreover, the patient experienced sudden cardiogenic shock with unstable hemodynamics, indicating that an invasive strategy should be the first choice in this setting to stabilize the patient’s conditions and to resolve the symptoms.
For patients who develop severe AI, re-sternotomy after LVAD implantation represents a very risky procedure, which includes left ventricular outflow tract occlusion, aortic valve replacement, aortic valve closure, and aortic patch closure, among other measures. These operations might provide a survival advantage over alternative treatments (16,23,24). However, open surgical correction can also increase the risks for ventricular damage or heart failure and hemorrhage, with the mortality rate being as high as 18% (25).
A transcatheter strategy would help avoid the surgical risks of re-sternotomy to correct AI in patients with LVAD. Regardless of whether a surgical intervention or transcatheter strategy is selected, the use of any intervention to occlude the aorta is not recommended, as this may increase the incidence of thromboembolism and sudden death caused by pump failure. TAVR, as one of the innovative approaches available (26-28), can be a first-line intervention for AI with the LVAD support and has been applied successfully elsewhere (29-31). In this case, a transcatheter self-expandable stent valve was deployed to correct AI and achieve functional recovery. Although TAVR has some beneficial characteristics, including its less-invasive nature and ability to preserve aortic valve function, it may be a challenging procedure to perform in this setting for the following reasons: (I) the absence of valvular calcification obscures the optimal landing position and seating area for the transcatheter valve, which may cause transcatheter valve migration; (II) the transcatheter heart valve prosthesis is prone to displacement in the ventricular direction when it is released under the negative pressure of the LVAD artificial pump; (III) an individual sizing limitation exists for the bioprosthetic valve; (IV) the patient’s autologous valve ring is susceptible to expansion. Despite these challenges, some countermeasures can be deployed: (I) one study showed that the valve could be up to 15% oversized relative to the aortic ring, so that the valve can be securely anchored to the valve ring (32); (II) a prosthetic valve can be positioned slightly higher than the aortic valve ring, forming an outflow space for the valve to move toward the ventricle; (III) the LVAD speed during implantation can be reduced; (IV) higher pressure can be applied during valve release and after dilatation to ensure full dilatation of the valve.
In this case, several measures were adopted to avoid valve displacement, including, a 30# prosthetic valve positioned at a level slightly over the aortic valve ring. Another 30# valve was positioned again within the prosthetic valve. The 26 and 32 mm balloons were used for high-pressure expansion to fix the overlapped valves on the valve ring. Different from the patients of LVAD-induced AI, aortic annulus calcification was common in original aortic valve dysfunction, prosthetic valve could be anchored stably on the calcification, so we do not recommend implant another prosthetic valve on the general AI patients.
Thus far, consensus has yet to be reached concerning the best strategy to use in managing AI with the LVAD support. There is limited number of published cases of successful TAVR (one series with an 89% 6-month survival and another series with a good 3-year outcome) (32,33). Indeed, more evidence is needed to define the treatment algorithm.
It has been noted that any delayed intervention to the point of systematic failure will predictably yield adverse outcomes. One report indicated that delayed surgical intervention for AI places the patient in an unstable condition (20). In this case, TAVR took less time to prepare and perform, brought less surgical trauma and a good clinical outcome, and thus benefitted the patient. Expeditious and effective intervention is the key to correcting severe AI after LVAD regardless of surgical intervention or transcatheter correction.
The new generations of valves with better anchoring mechanisms are undergoing clinical trials (34). One such valve is JenaValve (JenaValve Pericardial TAVR Aortic Regurgitation Study; NCT04415047), equipped with an anchor ring device to make the positioning more stable (35).
Questions
How can the occurrence of AI in patients with long-term LVAD be reduced?
Expert opinion 1: Daniel Wendt
Aortic regurgitation is a common problem in the long-term after LVAD implantation. Several concepts have been proposed to avoid or reduce the incidence of aortic regurgitation: blood pressure management including dedicated management of the pump itself. Moreover, new pump technologies offering more physiological pulsatile flows will induce better flow algorithms in the future. Speed optimization of the continuous-flow LVAD should be considered and evaluated. It has been shown that non-opening of the aortic valve at discharge after LVAD implantation is a predictor of developing aortic regurgitation in the long-term. Therefore, a dedicated echo prior to discharge including close follow-ups in the early postoperative period are mandatory.
Expert opinion 2: Calogera Pisano
In long term LVAD patients, we can improve AI using two methods:
- Medication optimization: it necessarily relieves congestive symptoms with diuretics and achieves adequate blood pressure control (mean arterial pressure goal <80 mmHg) using vasodilators and combination of many classes of agents (including beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and diuretics). The optimization of the blood pressure prevents progressive aortic dilation and heart failure. In cases of refractory heart failure, inotropes may be necessary.
- Pump parameters optimization: in order to promote atrioventricular opening and limit the AI, it is necessary to set the pump speed in a lower range for asymptomatic patients. If congestive symptoms occur and are not relieved by medication, the patient should undergo an echocardiograph-guided ramp study, as well as a right heart catheterization. Increasing pump speed increases left ventricular unloading and decreases left ventricular end-diastolic pressure (LVEDP). However, this will trigger a vicious cycle of complete closure of the aortic valve, resulting in an increase in AI due to elevated transvalvular pressure (TVP), and ultimately an increase in LVEDP. The optimal rotations per minute (RPM) are the RPM that can achieve hemodynamic optimization, defined as PCWP <18 mmHg, CVP <12 mmHg, CI >2.2 L/min/m2, ideally with intermittent atrioventricular opening and minimal mitral regurgitation.
If the patient is still symptomatic despite the maximal medical therapy and pump parameter optimization, surgical or percutaneous aortic valve interventions might be considered.
Expert opinion 3: Bartlomiej Perek
As it was found that systolic blood pressure when supported with LVAD could promote development/progression of secondary AI, an aggressive control of blood pressure was suggested to protect against this complication (36). A drop in blood pressure may decrease shear stress exerted on the wall of aortic root and then reduced LVAD-induced AI.
Expert opinion 4: Oliver Deutsch
Optimal medical management aimed at relieving congestive symptoms with diuretics and improving filling pressures with vasodilators and optimization of pump parameters, i.e. choosing lower pump speeds might support AV opening and eventually reduce the risk of developing AI. Patients with continuous flow devices seem to have more progressive AI than patients treated with pulsatile devices.
Is there a better anchoring method for TAVR in the management of secondary AI?
Expert opinion 1: Daniel Wendt
Various concepts have been suggested for better transcatheter valve anchoring in the setting of secondary aortic regurgitation. For example, new transcatheter valve substitutes will be developed in the future designed especially for aortic regurgitation. These valves will have other capabilities and features to cover larger aortic annuli and will also offer higher radial recoil forces to enable a better anchoring and valve fixation. Moreover, other concepts such as the Jena valve or J-valve offering “clipping” onto the native leaflets will be modified and updated especially to treat aortic regurgitation in a specific and optimized way. Other concepts start with the release of any kind of anchoring structure in the aortic annulus before starting the TAVR procedure (stent placement or first transcatheter valve serving as a landing zone). As a pre-requisite, all concepts of TAVR in the setting of prior LVAD implantation must be performed by other access routes than the transapical route as this has already been used for the LVAD device. In the special setting of LVAD implantation, one could potentially think about the implantation of an external aortic root annuloplasty ring or open band during LVAD implantation, which might not only present further dilation of the root, but also serve as a more rigid landing zone for future TAVR.
Expert opinion 2: Calogera Pisano
I think that you have already described in the discussion the countermeasures to adapt in order to better anchoring TAVR in the management of secondary AI: (I) the valve can be oversized relative to the aortic annulus, with one study suggesting a 15% oversizing, so that the valve can be firmly anchored to the aortic valve ring (28); (II) a prosthetic valve can be positioned slightly higher than the aortic valve ring, forming an outflow space for the valve to move toward the ventricle; (III) the LVAD speed during implantation can be reduced; (IV) higher pressure can be applied during valve release and after dilatation to ensure full dilatation of the valve.
Expert opinion 3: Bartlomiej Perek
The group of patients with previously implanted LVAD who underwent TAVI procedure for AI is very limited. Therefore, up to now there is no better anchoring method for TAVR in the management of LVAD-related secondary AI. We must adopt all recommendations for TAVR in pure native AI. In my opinion, the only possibility is to implant markedly oversized (by not exceeding 20%) newer generation percutaneous implants. Self-expanding prosthetic valves is preferred over balloon expandable valves because they can be oversized while preserving a low-risk of annular rupture by relying exclusively on their radial force at the level of the annular and ascending aorta. Moreover, there are some tricks during the procedure itself that can increase technical success of implantation such as the two pigtail catheters technique, avoiding balloon pre-dilatation and consideration of rapid pacing during deployment.
Expert opinion 4: Oliver Deutsch
A dedicated transcatheter heart valve prosthesis designed to treat aortic valve regurgitation might have superior anchoring properties especially in patients with the absence of device landing zone calcium.
Is there a way to identify the predictors of secondary AI before LVAD surgery?
Expert opinion 1: Daniel Wendt
First of all, a dedicated echo work-up prior to LVAD implantation is of utmost importance (vena contracta, etc.). It has been proven that any kind of aortic regurgitation prior to LVAD implantation comes with an increased risk of secondary aortic regurgitation in the long-term. Therefore, even in the case of mild aortic regurgitation prior to LVAD implantation simultaneous treatment of this concomitant finding should be considered. Although aortic valve opening after LVAD implantation is associated with a lower incidence of secondary aortic regurgitation, some authors proposed intraoperative commissural fusion of the aortic valve leaflets (AV leaflet stitching) during LVAD implantation to prevent secondary aortic regurgitation. In any case, the whole aortic root (sinuses, leaflets, bulbus, etc.) must be evaluated in detail prior to LVAD implantation to rule out any pathologies or abnormalities leading to a higher risk of secondary aortic regurgitation. Moreover, in a recent study by Kagawa et al. (37), it was shown that not only preoperative mild aortic regurgitation was a predictor for secondary aortic regurgitation, but also the duration of LVAD support (which is obvious) and interestingly, also in patients presenting with ischemic cardiomyopathy.
Expert opinion 2: Calogera Pisano
The most important risk factors associated with the development or progression of AI in patients with LVAD were advanced age, female, aortic valve closure, small body surface area, longer LVAD support time, small aortic root diameter, continuous flow pump, the location (2 cm above the sinotubular junction) and the angulation between the outflow graft and the ascending aorta (transverse angle ≥90°, coronal plane angle between 60° and 120°).
Expert opinion 3: Guillaume Goudot
I think it is important to focus on the mechanism—still incompletely understood at present—of aortic valve remodeling following LVAD placement. Dilatation of the sinus of Valsalva and alteration of the cusps play a role in AI.
So far, the factors identified as limiting AI seem to be the modification of LVAD settings to allow the aortic valve to open as often as possible, and the control of hypertension.
Expert opinion 4: Bartlomiej Perek
There are some predictors described in the literature of secondary significant AI before LVAD implantation. First of all, even mild preoperative AI should not be ignored. It would probably increase after surgery. Ischemic cardiomyopathy and mitral regurgitation of more than grade 2 or remained closed aortic valve at 1-month assessment after LVAD implantation are found independent predictors of secondary AI. In a systematic meta-analysis, Gasparovic et al. (38) claimed that older age, persistent aortic valve closure (again), female gender, and longer duration of continuous-flow LVAD support promoted development and progression of secondary AI. Continuous closure of the aortic valve after LVAD implantation, the most often cited risk factor for secondary AI development is found associated with higher right ventricular systolic work index (RVSWI). Recent study (39) employing computational models revealed that a place of outflow cannula could matter. Distance between outflow cannula-to-aortic root was shorter and shear stress exerted on the aortic root higher in patients who developed significant AI.
Expert opinion 5: Oliver Deutsch
Risk factors associated with development or progression of AI in LVAD patients include older age, female sex, absence of aortic valve opening, smaller body size (body surface area) and duration of LVAD support. Surgical aspects such as the placement and the angle between the outflow graft and the ascending aorta might also influence development of secondary AI.
Conclusions
Overall, a review of the case in this report suggests that severe AI with the LVAD support, particularly in the heart failing patients, warrants intervention. The appropriate, early treatment with TAVR provides the opportunity for functional recovery. Although we have experienced good outcomes with TAVR, the limited amount of evidence precludes recommendations concerning this procedure, and additional investigation is warranted.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1642/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1642/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1642/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Ethical Committee of TEDA International Cardiovascular Hospital [No. (2023)-0310-2]. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [Crossref] [PubMed]
- Frazier OH. Unforeseen consequences of therapy with continuous-flow pumps. Circ Heart Fail 2010;3:647-9. [Crossref] [PubMed]
- Jakovljevic DG, George RS, Nunan D, et al. The impact of acute reduction of continuous-flow left ventricular assist device support on cardiac and exercise performance. Heart 2010;96:1390-5. [Crossref] [PubMed]
- Pagani FD, Miller LW, Russell SD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol 2009;54:312-21. [Crossref] [PubMed]
- Noly PE, Pagani FD, Noiseux N, et al. Continuous-Flow Left Ventricular Assist Devices and Valvular Heart Disease: A Comprehensive Review. Can J Cardiol 2020;36:244-60. [Crossref] [PubMed]
- Acharya D, Kazui T, Al Rameni D, et al. Aortic valve disorders and left ventricular assist devices. Front Cardiovasc Med 2023;10:1098348. [Crossref] [PubMed]
- Sahni A, McIntyre EE, Pal JD, et al. Quantitative Assessment of Aortic Hemodynamics for Varying Left Ventricular Assist Device Outflow Graft Angles and Flow Pulsation. Ann Biomed Eng 2023;51:1226-43. [Crossref] [PubMed]
- Toda K, Fujita T, Domae K, et al. Late aortic insufficiency related to poor prognosis during left ventricular assist device support. Ann Thorac Surg 2011;92:929-34. [Crossref] [PubMed]
- Riebandt J, Schaefer A, Wiedemann D, et al. Concomitant cardiac surgery procedures during left ventricular assist device implantation: single-centre experience. Ann Cardiothorac Surg 2021;10:248-54. [Crossref] [PubMed]
- Zaidi SH, Minhas AMK, Sagheer S, et al. Clinical Outcomes of Transcatheter Aortic Valve Replacement (TAVR) Vs. Surgical Aortic Valve Replacement (SAVR) in Patients With Durable Left Ventricular Assist Device (LVAD). Curr Probl Cardiol 2022;47:101313. [Crossref] [PubMed]
- Baum C, Seiffert M, Treede H, et al. Concomitant transcatheter aortic valve and left ventricular assist device implantation. ASAIO J 2013;59:90-2. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Kormos RL, et al. Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transplant 2011;30:115-23. [Crossref] [PubMed]
- Calin E, Ducharme A, Carrier M, et al. Key questions about aortic insufficiency in patients with durable left ventricular assist devices. Front Cardiovasc Med 2022;9:1068707. [Crossref] [PubMed]
- Malick A, Ning Y, Kurlansky PA, et al. Development of De Novo Aortic Insufficiency in Patients With HeartMate 3. Ann Thorac Surg 2022;114:450-6. [Crossref] [PubMed]
- Jimenez Contreras F, Mendiola Pla M, Schroder J, et al. Progression of aortic valve insufficiency during centrifugal versus axial flow left ventricular assist device support. Eur J Cardiothorac Surg 2022;61:1188-96. [Crossref] [PubMed]
- Jain P, Meredith T, Adji A, et al. Spontaneous Oscillatory Left Ventricular-Aortic Uncoupling Under Continuous-Flow Left Ventricular Assist Device Support. Circ Heart Fail 2021;14:e007658. [Crossref] [PubMed]
- May-Newman K, Enriquez-Almaguer L, Posuwattanakul P, et al. Biomechanics of the aortic valve in the continuous flow VAD-assisted heart. ASAIO J 2010;56:301-8. [Crossref] [PubMed]
- Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157-87. [Crossref] [PubMed]
- Rajagopal K, Daneshmand MA, Patel CB, et al. Natural history and clinical effect of aortic valve regurgitation after left ventricular assist device implantation. J Thorac Cardiovasc Surg 2013;145:1373-9. [Crossref] [PubMed]
- Atkins BZ, Hashmi ZA, Ganapathi AM, et al. Surgical correction of aortic valve insufficiency after left ventricular assist device implantation. J Thorac Cardiovasc Surg 2013;146:1247-52. [Crossref] [PubMed]
- Krishnarao K, Krim SR. Management of hypertension in patients supported with continuous flow left ventricular assist devices. Curr Opin Cardiol 2023;38:281-6. [Crossref] [PubMed]
- Selzman CH. Commentary: Heart recovery: License to be offensive-on myocardial recovery following durable left ventricular assist device support. JTCVS Open 2021;8:6-7. [Crossref] [PubMed]
- Adamson RM, Dembitsky WP, Baradarian S, et al. Aortic valve closure associated with HeartMate left ventricular device support: technical considerations and long-term results. J Heart Lung Transplant 2011;30:576-82. [Crossref] [PubMed]
- Alkhouli M, Melito B, Ling FS. Percutaneous aortic valve closure for patients with left ventricular assist device-associated aortic insufficiency. Catheter Cardiovasc Interv 2016;88:1170-3. [Crossref] [PubMed]
- Rao V, Slater JP, Edwards NM, et al. Surgical management of valvular disease in patients requiring left ventricular assist device support. Ann Thorac Surg 2001;71:1448-53. [Crossref] [PubMed]
- Chatfield AG, Cheung A, Akodad M, et al. Transcatheter solutions for transcatheter aortic valve replacement dysfunction: is redo transcatheter aortic valve replacement a durable option? Ann Cardiothorac Surg 2021;10:571-84. [Crossref] [PubMed]
- Yeats BB, Yadav PK, Dasi LP, et al. Transcatheter aortic valve replacement for bicuspid aortic valve disease: does conventional surgery have a future? Ann Cardiothorac Surg 2022;11:389-401. [Crossref] [PubMed]
- Cahill TJ, Terre JA, George I. Over 15 years: the advancement of transcatheter aortic valve replacement. Ann Cardiothorac Surg 2020;9:442-51. [Crossref] [PubMed]
- Kherallah RY, Koneru S, Krajcer Z, et al. Hemodynamic outcomes after valve-in-valve transcatheter aortic valve replacement: a single-center experience. Ann Cardiothorac Surg 2021;10:630-40. [Crossref] [PubMed]
- Grohmann J, Blanke P, Benk C, et al. Trans-catheter closure of the native aortic valve with an Amplatzer Occluder to treat progressive aortic regurgitation after implantation of a left-ventricular assist device. Eur J Cardiothorac Surg 2011;39:e181-3. [Crossref] [PubMed]
- Menon AK, Dohmen G, Mahnken AH, et al. Successful combined procedure of HeartMate II left ventricular assist device implantation and minimally invasive transapical aortic valve replacement. J Thorac Cardiovasc Surg 2011;142:708-9. [Crossref] [PubMed]
- Iadanza A, D’Ascenzi F, Torrisi A, et al. TAVR in patients with left ventricular assist device: case report and literature review. Structural Heart 2019;3:11-7.
- Kar B, Prathipati P, Jumean M, et al. Management of Aortic Insufficiency Using Transcatheter Aortic Valve Replacement in Patients with Left Ventricular Assist Device Support. ASAIO J 2020;66:e82-6. [Crossref] [PubMed]
- Belkin MN, Imamura T, Fujino T, et al. Transcatheter aortic valve replacement in left ventricular assist device patients with aortic regurgitation. Structural Heart 2020;4:107-12.
- Poschner T, Werner P, Kocher A, et al. The JenaValve pericardial transcatheter aortic valve replacement system to treat aortic valve disease. Future Cardiol 2022;18:101-13. [Crossref] [PubMed]
- Patil NP, Mohite PN, Sabashnikov A, et al. Does postoperative blood pressure influence development of aortic regurgitation following continuous-flow left ventricular assist device implantation? Eur J Cardiothorac Surg 2016;49:788-94. [Crossref] [PubMed]
- Kagawa H, Aranda-Michel E, Kormos RL, et al. Aortic Insufficiency After Left Ventricular Assist Device Implantation: Predictors and Outcomes. Ann Thorac Surg 2020;110:836-43. [Crossref] [PubMed]
- Gasparovic H, Kopjar T, Saeed D, et al. De Novo Aortic Regurgitation After Continuous-Flow Left Ventricular Assist Device Implantation. Ann Thorac Surg 2017;104:704-11. [Crossref] [PubMed]
- Kasinpila P, Kong S, Fong R, et al. Use of patient-specific computational models for optimization of aortic insufficiency after implantation of left ventricular assist device. J Thorac Cardiovasc Surg 2021;162:1556-63. [Crossref] [PubMed]