
Extracorporeal membrane oxygenation (ECMO)-assisted surgery for traumatic bronchial rupture: a report of three cases
Highlight box
Key findings
• In patients with traumatic bronchial rupture, extracorporeal membrane oxygenation (ECMO)-assisted surgical treatment is of vital importance for their survival.
What is known and what is new?
• Traumatic tracheal rupture is a severe closed chest injury that often causes major respiratory and circulatory disturbances needing an emergency surgery.
• We have found that ECMO maintains good oxygenation because it acts as an artificial heart and lungs in patients with acute respiratory and circulatory failure.
What is the implication, and what should change now?
• Veno-venous ECMO can be useful adjunctive support in critically ill patients with traumatic bronchial rupture since it can allow more time for the physicians to resuscitate and stabilize the patient, and reduce the risk of emergency surgery.
Introduction
A traumatic bronchial rupture is one of the critical complications of a closed-chest injury, often following a major road traffic accident or trauma (1). It is a serious traumatic chest condition, often combined with severe hemopneumothorax, respiratory failure, and rib fractures. If not treated promptly, it can be life-threatening. Extracorporeal membrane oxygenation (ECMO) was initially used as a mechanical circulatory support for end-stage heart disease (2), and it plays a vital role in the resuscitation of patients with severe hypovolemia and respiratory failure after cardiac surgery (3). However, perioperative utilization of ECMO is also associated with increased risk of bleeding and acute kidney injury (3). With the development of ECMO devices and improvements in surgical placement techniques, this technology is gradually being used in clinical settings and the indications for it are increasing (4).
Veno-venous extracorporeal membrane oxygenation (VV-ECMO) is utilized in cases of primary respiratory failure that cannot be effectively managed with conventional medical treatments and mechanical ventilation. The primary objective of supporting VV-ECMO patients is to facilitate lung rest through the implementation of lung-protective ventilation strategies. Lung-protective ventilation aims to mitigate the triggering factors associated with ventilator-induced lung injury (VILI), including volume trauma, pressure trauma, atelectrauma, and biotrauma. In our patient cohort, the main indication for VV-ECMO was traumatic injury to the bronchi and trachea, resulting in the loss of autonomous breathing ability. On the other hand, venoarterial extracorporeal membrane oxygenation (VA-ECMO) is primarily employed for pre-existing heart failure, inadequate reperfusion, suboptimal myocardial protection during surgery, or technically challenging surgical procedures (5,6), It is evident that VV-ECMO would be more suitable for our patient. Overall, ECMO is currently available for several causes of cardiac and respiratory arrest, acute severe heart failure, and acute severe respiratory failure (7).
However, there is a lack of reports on the use of ECMO support for severe traumatic bronchial rupture patients with respiratory and circulatory failure (8,9). There are also no guidelines for the use of ECMO in the treatment of critically ill patients with traumatic bronchial rupture. Our hospital admitted 3 patients with a critical traumatic bronchial rupture from May 2019 to July 2021 who underwent successful emergency surgery with the assistance of VV-ECMO. In this report, we summarize our experience and discuss the feasibility of VV-ECMO application in the treatment of these patients. We present this article in accordance with the CARE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1783/rc).
Case presentation
Ethical statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients or their legal guardians or next of kin for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Case 1
Patient 1 was a 4-year-old boy who presented with a ruptured bronchus in the middle lobe of the right lung following a road traffic accident. He had repeated episodes of preoperative cardiac arrests, severe respiratory dysfunction, and difficulty maintaining oxygenation even with the support of ventilators.
Patient had a closed injury, presented with severe respiratory and circulatory dysfunction and underwent closed chest drainage before surgery. On admission, chest X-ray and computed tomography (CT) were performed, and pulmonary atelectasis on the affected side was found (Figure 1). The patient underwent bronchoscopy which revealed the presence of the bronchial ruptures (Figure 1). Preoperative bedside fiberoptic bronchoscopy suggested right middle lobe bronchial dissection.
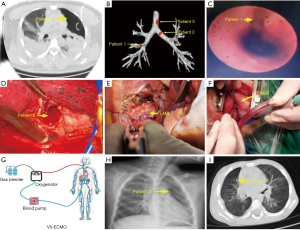
Patient 1 had 3 cardiac arrests before that led to the decision to implant the ECMO. He had another cardiac arrest after the ECMO implantation. A blood gas analysis revealed that the patient had hyperkalemia, which was immediately corrected. No further cardiac arrests occurred. ECMO flow was stable with a satisfactory oxygen saturation of 98–100%.
VV-ECMO with Medtronic cannulas (Minneapolis, MN, USA) and Maquet machines (Getinge, Gothenburg, Sweden) was implanted (Figure 1). Outflow cannula was inserted in the right femoral vein and inflow cannula in the right internal jugular vein (under bedside ultrasound guidance); ECMO flow rate ranged from 40 to 100 mL·kg−1·min−1.
The ECMO tubes were flushed with 0.9% saline + heparin (1 mL of saline: 1 U of heparin) and then pre-filled with 5 U of the corresponding blood type of concentrated red blood cells. For case 1, without imaging guidance, the right femoral vein was cannulated, while under bedside ultrasound guidance, the right internal jugular vein was cannulated.
Patient 1 underwent right lobectomy combined with right middle bronchoplasty. Posterolateral thoracotomy on the affected side through the 4th or 5th rib space was performed. After entering the thoracic cavity, the inferior pulmonary ligament was dissected first, and the chest was explored (Figure 1). The right middle lung bronchus was found severely fragmented near the root, and the underlying bronchus was also severely fragmented with an indistinct tissue pattern. Consequently, the right middle lobe was resected. ECMO was maintained for 123 h; the postoperative mechanical ventilation was required 8 days.
Case 2
Patient 2 was a 41-year-old male who experienced a left main bronchial rupture as a result of a traffic accident. He was first resuscitated at a local hospital and then transferred to our hospital (nearly 2 days after his injury) for chest tightness, shortness of breath, and acute respiratory failure, combined with severe hypoxemia that could not be managed with a ventilator before the operation.
Patient 2 also had closed injuries. He underwent standard preoperative work-up. Preoperative bedside fiberoptic bronchoscopy suggested “left main bronchial dissection”.
Patient 2 also underwent emergency VV-ECMO support surgery and underwent left bronchial rupture repair. The ECMO was set as follows: initial speed of 3,240 rpm, oxygen flow rate of 5 L/min, and blood flow rate of 4.17 L/min. Under the mentioned support mode, the oxygen saturation remained at 95%. The ventilator mode was adjusted to Bipap, with a positive end-expiratory pressure (PEEP) of 2 cmH2O, peak inspiratory pressure (PIP) of 13 cmH2O, oxygen concentration of 100%, and the oxygen saturation was maintained at around 90–95%. The severed ends were anastomosed by trimming the bronchus using 3-0 Vicryl or 4-0 PDS sutures: one with continuous sutures and the other with interrupted full sutures (Figure 1). A small portion of the visceral pleura was used to cover and reinforce the anastomosis to prevent bronchopleural fistulae. After the chest cavity was flushed following the bronchial anastomosis, the lung was inflated under pressure. The anastomosis was then observed to ensure that there was no air leak, and the affected lung was reopened.
ECMO was explanted after 98 h. The postoperative mechanical ventilation was used for 5 days, and the length of the intensive care unit (ICU) stay was 7 days.
Case 3
Patient 3 was a 4-year-old boy who had a longitudinal rupture of the main bronchus caused by a blunt injury by a falling object. He presented with severe hypoxemia, severe mediastinal and subcutaneous emphysema, and could not be mechanically ventilated before surgery. Preoperative bedside fiberoptic bronchoscopy suggested longitudinal main tracheal dissection. After implantation of VV-ECMO, patient 3 underwent repair surgery for longitudinal main tracheal rupture, and the surgical procedure was analogous to that of the patient 2.
The patient required ECMO for 76 h and postoperative bronchoscopy revealed a longitudinal tear in the main tracheal membrane above the bulge during. However, since there were no signs of air leakage, and considering it was an incomplete laceration, we decided for conservative management and monitoring. On a 4th postoperative day, a repeated tracheoscopy was performed and it showed that the fissure in the main trachea above the bulge has healed. VV-ECMO was removed and the patient was successfully extubated after 36 h. The patient was discharged from the ICU 6 days after surgery.
Treatment and follow-up
All three patients were admitted to the ICU for postoperative ECMO maintenance and started on a heparin micropump maintenance anticoagulation 24 h postoperatively, maintaining an activated partial thromboplastin time (APTT) of 50–65 s, with APTT measured at 2–3-hour intervals. Platelets were maintained above 50×109/L. There was no postoperative hemorrhage and patients were awakened within 48 h postoperatively.
All the patients had experienced no postoperative complications. Furthermore, the distance between the bronchial stump and the tracheal ramus was less than 2 cm in all 3 patients. The postoperative mechanical ventilation time was on average 6.5 (interquartile range, 5–8) days, duration of ECMO support was 105.5 (interquartile range, 98–123) h, and duration of surgery was 155 (interquartile range, 135–180) min. Of note, patient 3 had a longitudinal tear in the tracheal membrane above the ramus during bronchoscopy on the first postoperative day, but there were no signs of air leakage in ventilator parameters or on chest drainage. We suspected an incomplete tear, and consequently, the airway pressure was reduced. After 24 h of monitoring, a further bronchoscopy revealed that the laceration had closed, and the patient was withdrawn from ECMO on the third postoperative day.
All patients had no postoperative complications such as bronchopleural fistula or bleeding. Postoperative review showed good lung re-expansion on the affected side in all patients (Figure 1). There were no deaths in this group, and all patients were discharged after being cured, allowing them to engage in general physical activities. Follow-up ranged from 1 month to 2 years, and chest CT scans did not indicate significant bronchial stenosis in the patients.
iMDT discussion
Discussion among physicians from Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University
Case 1
Department of Thoracic Surgery
During the surgical procedure, it was found that the patient had a rupture of the right middle lobe bronchus and one branch of the right middle pulmonary artery. After the surgical treatment, we need to pay special attention to postoperative pulmonary function rehabilitation, lung ventilation, and closely monitor the patient’s chest X-ray.
Department of Pediatrics
For a 4-year-old child, it is necessary to limit fluid intake and be mindful of the risks of intracranial hemorrhage and edema. Intravenous administration or continuous infusion of concentrated sodium can be used to prevent the occurrence of cerebral edema. Additionally, the dosage of intravenous lipid emulsion should be adjusted, with a focus on maintaining internal homeostasis.
Department of Neurology
The patient is in a sedated state, which makes it difficult to accurately assess their consciousness. However, both pupils exhibit light reflexes and no pathological signs were observed. If the condition allows, a repeated head CT scan can be performed. Due to the prolonged period of hypoxia after cardiopulmonary resuscitation, there is a concern for cerebral edema. Considering the patient’s young age, hypertonic saline may be used to mitigate cerebral edema.
Case 3
Department of Thoracic Surgery
Patient 3 had tracheal fissure detected by bronchoscopy before operation, which has been discussed in our department. With the help of VV-ECMO, the child underwent “exploratory thoracotomy plus repair of tracheal rupture” on August 29, 2021. After the repair, no air leakage was found in the bulging lung during the operation. Bronchoscopy was performed during and after the operation, and no fissure was found at that time. At present, it is found that the cause of tracheal dehiscence may be related to the damage of tracheal tissue and structural fragility caused by violent trauma in children, and it may be torn again after repair. If the fissure persists, it may take a long time to repair itself, and the secretions in the airway may spread to the mediastinum along the fissure, which poses the risk of mediastinal infection. It is suggested to open the chest again for tracheal rupture repair.
Department of Respiratory Medicine
The doctor repeatedly reviewed the video of the patient’s bronchoscopy examination. It is possible that the patient has a tracheal rupture, but there are no air bubbles leaking from the closed suction drains in both sides of the chest at present. The parameters of the ventilator indicate signs of air leakage. It suggests that the patient may have a partial-thickness tracheal rupture. If the patient has a partial-thickness tracheal rupture, it is possible for the tracheal tissue to heal on its own. If necessary, consideration can be given to the placement of a covered tracheal stent. If the patient does not have a full-thickness rupture, surgical intervention may be required.
Department of Pediatrics
We agree with respiratory physicians that if endotracheal stenting is needed, the diameter of the trachea may be 0.7–1.5 cm. The size of the trachea can be measured according to CT imaging examination, and the tracheal stent can be customized according to the size of the trachea. At present, a child is hemodynamically stable, and it is necessary to improve nutritional support to promote wound healing. It is recommended to consult the Department for Nutrition, evaluate nutritional status, and provide suitable enteral nutritional supplements.
Department of Vascular Surgery
The patient is currently treated with VV-ECMO, with a little blood oozing at the cannulation site, which does not need special treatment for the time being. The right femoral vein cannulation was performed with an open incision.
Several issues regarding the diagnosis and treatment of these patients were further discussed as follows
- What do you think the indications of ECMO in traumatic tracheal rupture include?
Expert opinion 1: Alexander Kaserer. There are several indications and reasons for using ECMO in patients suffering from a traumatic tracheal rupture. In such cases, the endotracheal intubation can be difficult or even impossible due to tracheal injury. ECMO may be necessary to maintain gas exchange till a secure airway is established. A traumatic tracheal rupture results in significant air leakage, leading to severe respiratory distress with hypoxemia. Conventional mechanical ventilation techniques may be unable to adequately oxygenate the patient due to the disruption of the trachea. Here, ECMO provides a sufficient oxygenation and carbon dioxide control without high airway pressures of mechanical ventilation. This reduces the risk of an additional barotrauma. Traumatic tracheal rupture can be associated with significant bleeding or other concomitant injuries, leading to hemodynamic instability. ECMO may help to hemodynamically stabilize the patient, if a VA or a veno-veno-arterial (VVA) ECMO therapy is performed. However, ECMO requires anticoagulation which increases the risk of additional bleeding complications, so careful monitoring of anticoagulation levels and a balanced approach to anticoagulation management are essential. ECMO may be initiated to stabilize the patient before undergoing surgical repair of the tracheal injury. This approach provides a controlled environment for the surgical team to work on repairing the trachea. After the surgery, ECMO is a useful bridge to recovery, allowing time for the tracheal injury to heal, or as a bridge to a more definitive treatment plan, such as tracheal reconstruction or stent placement.
Expert opinion 2: Dr. Davorin Sef. ECMO can be applied as intraoperative support during the surgical repair of traumatic tracheal or bronchial rupture in patients who developed refractory hypoxemia and hemodynamic instability. Furthermore, VV-ECMO may be considered for supportive care in patients with severe acute respiratory failure after traumatic tracheal or bronchial rupture considering increased risk of bleeding and other ECMO-related complications. - What are the precautions for children to retain ECMO?
Expert opinion 1: Alexander Kaserer. Several precautions and considerations must be considered to ensure the safe and effective use of ECMO in pediatric patients. ECMO should only be administered by a highly specialized and experienced medical team including pediatric intensivists or anesthesiologist, perfusionists, and pediatric surgeons. ECMO provides cardiac and/or respiratory support, but it does not eliminate the need for mechanical ventilation. Children may still require mechanical ventilation to support their respiratory needs while on ECMO. The underlying cause of the failure should be treatable, and the expected benefit of ECMO should outweigh the potential risks. Moreover, ECMO treatment requires anticoagulation to prevent clot formation within the circuit. Children can be more susceptible to bleeding complications, so careful monitoring of anticoagulation levels and a balanced approach to anticoagulation management are essential. ECMO can be associated with various complications, including bleeding, thrombosis, vascular injury, and organ dysfunction. Prompt recognition and management of these complications are vital. Infections are a significant concern with ECMO. Strict infection control measures, including sterile technique during cannulation and circuit management, are crucial to minimize the risk of bloodstream infections. Finally, providing emotional support and education to the child’s family is essential. ECMO can be a stressful and challenging experience, and families should be kept informed about their child’s condition and progress.
Expert opinion 2: Dr. Davorin Sef. The decision to retain ECMO should be discussed within a multidisciplinary team including pediatric surgeons, pediatric intensivists, perfusionists, and respiratory physicians. This decision should be made considering oxygenation, adequate circulation and organ perfusion on one hand, and risks of ECMO-related complications on the other hand. - Please comment on the treatment of these patients.
Expert opinion 1: Alexander Kaserer. Traumatic tracheal rupture is a rare but potentially life-threatening condition. To initiate ECMO treatment in patients suffering from a traumatic tracheal rupture is a complex decision that requires careful assessment by a multidisciplinary team of medical professionals. Careful patient selection is crucial. The decision to initiate ECMO should be based on the patient’s overall clinical condition, the severity of the tracheal injury, concomitant injuries, and the likelihood of achieving a favorable outcome. Especially ECMO in children is a complex and high-risk intervention. ECMO should only be administered by a highly specialized and experienced medical team. The management of pediatric ECMO patients should be guided by established protocols and best practices to maximize the chances of a favorable outcome. Once the patient is stabilized, a well-planned strategy for weaning from ECMO and decannulation should be in place to transition the patient back to conventional therapies as their condition improves.
Expert opinion 2: Dr. Davorin Sef. As discussed above, these patients underwent successful surgical treatment of traumatic tracheal or bronchial rupture with the support of ECMO due to severe acute respiratory failure with or without hemodynamic instability.
Summarize the discussion
Traumatic bronchial rupture is an uncommon type of critical and serious thoracic trauma, most often caused by major road traffic accidents (10). Early clinical manifestations are mainly chest tightness, dyspnea, hemopneumothorax, and hemoptysis, which can be life-threatening. The possible pathogenesis of traumatic bronchial rupture is as follows: (I) following chest compression, the antero-posterior diameters become smaller, causing the lung to pull the bronchus laterally, resulting in rupture; (II) tracheobronchial rupture is caused by a sudden rise in pressure due to the momentary closure of the vocal cords under the increased intrahoracic pressure; (III) sudden deceleration of the body and lungs, with large shearing forces at a fixed point of the trachea, can increase the internal bronchial pressure, leading to its fracture (11). There are 2 types of bronchial rupture in the clinical setting: Type I, where the bronchial rupture is connected to the pleural cavity, mainly manifesting as tension pneumothorax and hemothorax; Type II, where the ruptured bronchial opening is not connected to the pleural cavity and the pneumothorax is not obvious, mainly manifesting as mediastinal emphysema. Currently, the treatment of traumatic bronchial rupture is largely based on surgical intervention (12). A literature review that was performed on this topic suggested that primary surgical repair should be considered when the patient’s vital signs are stable (13). In patients with chest tightness and dyspnea following trauma, surgery should be performed as soon as they are found to have a tracheal or bronchial rupture. However, these patients are often admitted to the hospital as a result of trauma and are likely to have acute respiratory and circulatory failure. Surgical treatment at this time often poses a significant risk of mortality.
The main goal of VV-ECMO is to promote lung rest through lung-protective ventilation. Lung-protective ventilation aims to reduce triggering factors associated with VILI, such as volume trauma, pressure trauma, atelectrauma, and biotrauma. It also helps critically ill patients recover, providing sufficient time for definitive treatment. It can be used as a treatment option for patients with acute biventricular heart failure complicated by respiratory failure, especially as adjunctive therapy in cases of cardiac arrest (5,14-16).
There are 2 common ECMO configurations. A is VA where the femoral artery and the vein are cannulated. The other is the VV approach, where the femoral vein and the internal jugular vein are cannulated (5). In this report, our team successfully applied VV-ECMO to provide adjunctive support to 3 critically ill patients with bronchial rupture and concomitant circulatory and respiratory failure while safely completing the emergency surgical procedure. In summary, we presented the feasibility of the ECMO application in the case of traumatic tracheal or bronchial rupture. VV-ECMO for adjunctive support in critically ill traumatic bronchial rupture patients can allow more time to resuscitate and stabilize the patient and can reduce the risk of anesthesia and emergency surgery (17). However, further studies with larger cohorts and long-term follow-up are needed to further assess its value in this field.
Conclusions
We presented the feasibility of the VV-ECMO in the case of traumatic bronchial rupture. VV-ECMO for adjunctive support in critically ill traumatic bronchial rupture patients can allow more time to resuscitate and stabilize the patient and can reduce the risk of emergent surgery. However, studies with larger cohorts and long-term follow-up are needed to further assess its value in this field.
Acknowledgments
The authors thank Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University for providing the research environment.
Funding: The present study was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1783/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1783/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1783/coif). A.K. has received support from Bayer AG (Switzerland) and CSL Behring GmBH (Switzerland) for lecturing. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients or their legal guardians or next of kin for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harvey-Smith W, Bush W, Northrop C. Traumatic bronchial rupture. AJR Am J Roentgenol 1980;134:1189-93. [Crossref] [PubMed]
- Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017;63:60-7. [Crossref] [PubMed]
- Sef D, Verzelloni Sef A, Mohite P, et al. Utilization of extracorporeal membrane oxygenation in DCD and DBD lung transplants: a 2-year single-center experience. Transpl Int 2020;33:1788-98. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. Erratum in: Lancet 2009;374:1330. [Crossref] [PubMed]
- Wrisinger WC, Thompson SL. Basics of Extracorporeal Membrane Oxygenation. Surg Clin North Am 2022;102:23-35. [Crossref] [PubMed]
- Ricciardi G, Putman LM, Hazekamp MG. Repair of traumatic avulsion of the right bronchus in children using extracorporeal membrane oxygenation support. Interact Cardiovasc Thorac Surg 2021;32:834-6. [Crossref] [PubMed]
- Zhang Y, Luo M, Wang B, et al. Perioperative, protective use of extracorporeal membrane oxygenation in complex thoracic surgery. Perfusion 2022;37:590-7. [Crossref] [PubMed]
- Chu X, Chen W, Wang Y, et al. ECMO for paediatric cardiac arrest caused by bronchial rupture and severe lung injury: a case report about life-threatening rescue at an adult ECMO centre. J Cardiothorac Surg 2022;17:142. [Crossref] [PubMed]
- Liu C, Lin Y, Du B, et al. Extracorporeal membrane oxygenation as a support for emergency bronchial reconstruction in a traumatic patient with severe hypoxaemia. Interact Cardiovasc Thorac Surg 2014;19:699-701. [Crossref] [PubMed]
- Sanli M, Isik AF, Tuncozgur B, et al. Successful repair in a child with traumatic complex bronchial rupture. Pediatr Int 2010;52:e26-8. [Crossref] [PubMed]
- Baumgartner F, Sheppard B, de Virgilio C, et al. Tracheal and main bronchial disruptions after blunt chest trauma: presentation and management. Ann Thorac Surg 1990;50:569-74. [Crossref] [PubMed]
- Gabor S, Renner H, Pinter H, et al. Indications for surgery in tracheobronchial ruptures. Eur J Cardiothorac Surg 2001;20:399-404. [Crossref] [PubMed]
- van Roozendaal LM, van Gool MH, Sprooten RTM, et al. Surgical treatment of bronchial rupture in blunt chest trauma: a review of literature. J Thorac Dis 2018;10:5576-83. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- Munshi L, Walkey A, Goligher E, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med 2019;7:163-72. [Crossref] [PubMed]
- Burkhoff D, Sayer G, Doshi D, et al. Hemodynamics of Mechanical Circulatory Support. J Am Coll Cardiol 2015;66:2663-74. [Crossref] [PubMed]
- Swol J, Cannon JW, Barbaro RP, et al. Extracorporeal Membrane Oxygenation in Trauma. ASAIO J 2022;68:e62-3. [Crossref] [PubMed]


