Successful repair of acquired intrathoracic nonmalignant tracheoesophageal fistulas using thoracoacromial artery perforator flap through a midsternal incision approach: a report of three cases
Highlight box
Key findings
• Repair of tracheoesophageal fistula (TEF) using a thoracoacromial artery perforator flap (TAPF) through a midsternal incision approach is an effective, safe surgical treatment.
What is known, and what is new?
• TEF is a challenging surgical problem and can be a life-threatening condition due to its complications. The treatment strategy for TEF is controversial, and the mortality, morbidity and complication cannot be ignored.
• Here, we introduce our innovative experience repairing TEF with a TAPF. We sought a midsternal approach that could provide excellent exposure and manipulation room. We chose the TAPF, which has a constant artery perforator, sufficient pedicle length, wide territory and appropriate tissue thickness of the flap, making it suitable for reconstruction of the esophagus in the narrow mediastinal space.
What are the implications, and what should change now?
• Repair of TEF using TAPF through a midsternal incision approach is a promising operation that offers a more simple procedure and has fewer complications.
Introduction
Acquired intrathoracic nonmalignant tracheoesophageal fistulas (TEFs) are rare and challenging surgical problem. TEF can be a life-threatening condition due to severe pulmonary complications and poor nutrition (1,2). Surgical treatment is effective for most patients undergoing operative repair, which provides a curative opportunity compared with endoscopic stenting, Once the diagnosis is confirmed, the surgical treatment should be the first consideration (3,4). TEF repair surgeries can be complex and require delicate surgical skills. The procedures involve accessing and repairing both the trachea and the esophagus, which can be challenging due to their proximity and delicate structures. In cases of fistula formation secondary to esophagectomy, the previous surgery and anastomosis leak can cause severe inflammation and fibrosis, further increasing the difficulty of the surgery. Complicated cases may require specialized techniques, such as tissue grafting or reconstructive surgery, which require a good surgical field of view and well-designed strategies (3). Several differing surgical management strategies have been reported (5,6). However, postoperative mortality and complication morbidity cannot be ignored in recent studies. The early complications of surgical treatment can be as high as 62.5%. Among them, esophageal stricture is the most common postoperative complication, occurring in 42–54% of patients, and anastomosis leakage occurs at a rate of 22.7–26%. The recurrence rate of fistula is about 5%, and the mortality rate can be as high as 29.4%, with 76.2% of deaths occurring in the early postoperative period (5,7-11).
The thoracoacromial artery perforator flap (TAPF) is considered a workhorse flap by many otolaryngology-head and neck surgeons because of its versatility and reliability (12). There is a constant thoracoacromial artery perforator present in the space between the clavicular and sternocostal heads of the pectoralis major muscle. The appropriate flap thickness, sufficient pedicle length and territory make it a suitable donor-site area for reconstruction of the esophagus in a narrow mediastinal space.
This study reports the first time that a TAPF has been used for the repair of intrathoracic acquired tracheoesophageal fistulas (aTEFs) through a midsternal incision approach, which provides a clear surgical field of view, reliable reconstruction, and no serious complications during the perioperative period. After 6 months of follow-up, no recurrence has been observed. At the same time, the problem of postoperative esophageal stricture has been solved by enlarging the esophageal lumen through skin flaps, improving the quality of postoperative life. We present this article in accordance with the CARE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1128/rc).
Case presentation
Patients
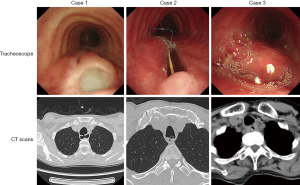
Three consecutive surgical repairs with TAPFs was performed between July 2021 and January 2022. None of the patients’ physical examinations showed significant abnormalities, all patient received computed tomography (CT) scan and tracheoscopy examination to assess the fistulas (Figure 1). The patient characteristics are described below and listed in Table 1. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent form is available for review by the editorial office of this journal.
Table 1
| Variables | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age (years) | 40 | 58 | 63 |
| Gender | Female | Female | Male |
| Etiology of fistula | Complication of prolonged mechanical ventilation | Complication of prolonged mechanical ventilation | Complication of previous esophageal surgery |
| Location of fistula | Mid trachea | Proximal trachea | Mid trachea |
| Size of fistula (cm) | 3.5 | 3.0 | 1.5 |
| Interval between initial event and surgical repair | 3 years | 6 months | 6 months |
| Esophageal anastomotic leak post operation | Yes | Yes | No |
| Length of stay (days) | 52 | 49 | 56 |
Case 1
A 40-year-old female visited our hospital with a history of aTEF for 3 years. She suffered from progressive muscle weakness and respiratory muscle paralysis caused by Guillain-Barré syndrome 3 years ago. At that time, she received approximately 4 weeks of hospitalization in the neonatal intensive care unit (NICU) of a medical institution in her residing area, where she underwent tracheotomy and mechanical ventilation treatment. After the primary disease improved, she suffered from shortness of breath and coughing while swallowing, then she was diagnosed to suffer aTEF and received non-surgical treatment, however there was no significant improvement and the symptoms still remained recurring. Chest CT examination showed the existence of aTEF and fiberoptic bronchoscope showed that there existed aTEF located in the mid trachea and was about 3.5 cm in diameter. She received nutrition support by a nasogastric tube without oral feeding, and the patient’s malnutrition was corrected before operation, and the Eastern Cooperative Oncology Group (ECOG) score was 1.
Case 2
A 58-year-old female experienced consciousness impairment and respiratory dysfunction due to a cerebral stroke caused by a ruptured intracranial aneurysm and received intracranial aneurysm clipping and vascular reconstruction 8 months ago, then she was admitted to the NICU of a medical institution in her residing city for approximately 8 weeks, during which she also underwent tracheotomy and mechanical ventilation treatment. After that, she suffered from shortness of breath, coughing while swallowing and recurrent pneumonias, and was diagnosed to suffer aTEF. Chest CT and fiberoptic bronchoscope showed that aTEF located in the proximal trachea and was about 3.0 cm in diameter. She received nutrition support by a nasogastric tube without oral feeding either, and antibiotic therapies for recurrent lung infection to ensure the malnutrition was corrected, and there was no pulmonary infection. The ECOG score was 3 before surgery.
Case 3
A 63-year-old male was admitted with a history of aTEF for 6 months caused by complication of previous esophagectomy for esophageal cancer. The patient developed an esophagogastric anastomotic leak after the previous operation, which progressed into a TEF. After diagnosis was confirmed, surgical treatment was considered as the preferred treatment option. Moreover, a 6-month active observation was taken before surgical management in order to help healing and improve surgical dissection by allowing decrease in inflammation at the operation field, and kept nutrition support by a nasogastric tube. Chest CT and fiberoptic bronchoscope showed that aTEF located in the mid trachea and was about 1.5 cm in diameter. The degree of esophageal stenosis in the case was severe and can only accommodate the passage of a nasogastric feeding tube. His ECOG score was 2 before surgery.
Therapeutic intervention
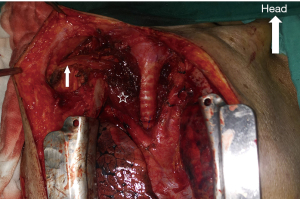
The patient was placed in a supine position with the neck extended, and he or she was placed under general anesthesia with a single lumen endotracheal tube. The surgical field was prepped in a standard sterile fashion, and a midsternal incision or a partial splitting of the sternum was made for this procedure according to operator’s experience and preference as described elsewhere (6). The trachea was exposed after the thymus and anterior mediastinal fat were resected, and the left innominate vein was transected. The bilateral recurrent laryngeal nerves were identified and protected.
Bronchoscopic transillumination was applied to locate the fistula after the trachea was mobilized. The trachea was divided at the middle point of the TEF. The cuff of the indwelling tube was deflated prior to making the incision and then withdrawn once the distal tracheal lumen had been identified. Cross-field ventilation was established by intubating the distal tracheal endotracheal tube in the surgical field and connecting it to sterile ventilator circuit tubing, which was previously passed off to the anesthesiologist.
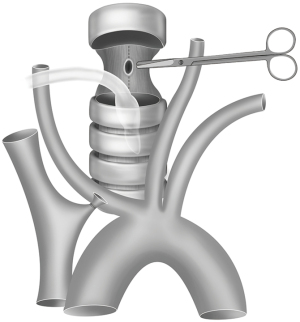
The esophagus (or the thoracostomach) can be dissected and divided much more easily after the trachea is incised and pulled to the cranial and caudal sides. The esophagus (or the thoracostomach) was longitudinally cut beyond both ends of the stenosis and fistula until healthy muscularis and mucosa layers were obtained. It was used for further repair as the rear wall of the reconstructed esophagus (Figure 2).
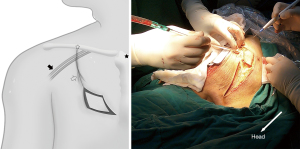
An arc, the body surface reflection of the thoracoacromial artery perforator, was drawn from the midclavicular to the third intercostal, as the center of the circle is the midpoint of the manubrium sterni, and the radius was from the midpoint of the manubrium sterni to the midpoint of the clavicle (Figure 3). Dissection started with an incision along this line and deep to the pectoralis major muscle until the dominant perforator emerging from the space between the clavicular and sternocostal heads of the pectoralis major muscle was encountered. As the perforator was localized and was freed from its fascial strands, the desired mobility was obtained. The skin island was designed to adequately match the size and shape of the esophageal defect.
Once the adequate mobility of the flap and its pedicle length were ascertained to be sufficient, the flap was passed under the clavicular head of the pectoralis major and through a subcutaneous tunnel above the clavicle and the sternocleidomastoid into the superior mediastinum for repair (Figure 4). Sometimes, a part of the sternocleidomastoid need to be incised to ensure a tensionless reconstruction. The donor site was closed directly with the ability for subcutaneous drainage.
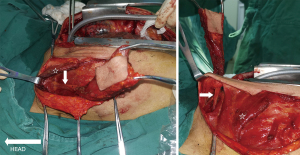
A nasogastric tube was inserted to the distal end of the esophageal defect with direct vision to prevent narrowing of the esophageal lumen. With the trachea retracted, the flap was placed on the esophagus with the skin side toward the esophageal lumen. This allows the skin to serve as a substitute for mucosa, providing protection against infection and acid. The flap served as the front wall of the reconstructed esophagus using interrupted 2-0 Ethibond sutures. After reconstruction, the esophagus and trachea were separated by the musculocutaneous flap (Figure 5).
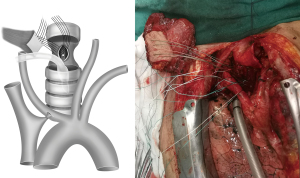
Trachea repair strategies can vary depending on the tension of the anastomosis, which is determined by the size of the tracheal fistula and the thickness of the musculocutaneous flap. For cases with small fistulas, segmental resection of the diseased trachea would be appropriate to achieve a no-tension anastomosis. In other cases in which the anastomotic tension was significantly increased because of a large fistula or a long trachea going above the interposition musculocutaneous flap, we recommend stitching the tracheal defect directly with longitudinal continuous 3-0 Prolene sutures and then anastomosing the tracheal incision end to end (Figure 6). No tracheal segments were removed in this way, and a tension-free anastomosis could be performed. The main drawback is that the loss of the tracheal membrane leads to a slight narrowing of the trachea. However, no signs or symptoms of dyspnea were observed in our patients, indicating that it is not a serious problem.
Two drains on either side of the anastomosis and a chest tube were placed, and the incision was closed in layers.
A postoperative healing time of 3 weeks is needed. Then, chest CT, upper gastrointestinal angiography and gastroscopy should be performed to assess the esophageal anastomosis. Subsequently, the patient can resume oral intake if the anastomosis has healed well, and the drain tube can be removed.
Follow-up and outcomes
Repair of TEFs using a TAPF through a midsternal incision approach was completed in 3 patients. All patients were extubated in the postoperative unit without an intensive care unit (ICU) stay. Two patients had complications of esophageal anastomotic leakage, manifesting as transitory fever and murky drainage. However, they eventually healed well after nonsurgical treatment. No tracheal anastomotic leakage was observed, and the average length of hospitalization was 42 days.
All patients were allowed general diet oral feeding at the time of discharge. At 6 months postoperatively, all patients underwent evaluations that included medical history assessment, physical examination, chest CT scan, bronchoscopy, and gastroscopy. There are no mortality, esophageal stenosis, dyspnea or other complications were observed.
International multidisciplinary team (iMDT) discussion
Discussion among physicians from West China Hospital
Department of thoracic surgery
Acquired intrathoracic nonmalignant TEFs can result from complications of esophageal surgery (13), trauma, foreign bodies (14), granulomatous infection, erosion of indwelling airways or esophageal stents and prolonged mechanical ventilation (1). Repair, especially secondary to esophageal anastomotic leakage, can be a challenging surgical problem.
Endoscopic esophageal self-expanding stent and tracheal Y-stent placement can be useful as a temporizing measure for patients who are ventilator dependent (2). However, endoscopic treatment leads to a TEF closure success rate of 45.5% to 68.8% (15,16); additionally, due to the disruption of respiratory epithelial function, stent erosion and granulation growth, it plays a limited role in patients with acquired benign fistulas. Successful surgical management strategies for TEFs have been reported. However, the reported operative mortality was 5.7% to 29.4% (5,7-10), and more than 50% of patients had complications (5,11). Respiratory failure and pneumonia were the most common postoperative complications. Furthermore, postoperative recurrence and postoperative esophageal stenosis must be resolved.
Right thoracotomy is usually chosen; however, the following three reasons make the fistula difficult to expose and repair through this approach. The first reason is that the fistula is often located at the top of the rib cage, which is also the deepest position of the operation area, leading to unsatisfactory exposure. The second is that, in many patients who undergo previous esophageal surgery, there is an anastomotic leak and much more inflammation in the right chest, and desmoplasia in the thoracic cavity and around the fistula makes the procedure difficult and complex. The last reason is that the space of the mediastinum is narrow, the esophagus is tightly attached to the trachea, and the plane of the fistula is parallel to our sight. In some cases, the left wall of the fistula might even be totally obscured, making it very difficult to suture the fistula and flap. Furthermore, the protection of the left recurrent laryngeal nerve at this condition poses considerable challenge, which may be a significant factor leading to long-term vocal cord paresis/paralysis in some patients (8). For all these reasons, we sought a new approach that could provide excellent exposure and manipulation room.
The midsternal incision repair procedure seems to be a satisfactory solution. Using this strategy, we could approach the trachea and the esophagus (or the thoracostomach) through the unoperated space of the anterior mediastinum. There were no obstacles from the previous operation, and there was no scars or adhesions. The operation was also much easier. The esophagus (or the thoracostomach) and bilateral recurrent laryngeal nerves were exposed clearly after the trachea was incised and pulled toward the head and caudal separately. Thus, postoperative hoarseness and the incidence of lung complications could be reduced. The fistula could be accessible and easily repaired with a direct view.
Esophageal anastomotic leakage is the most serious complication of this procedure, and fever and murky drainage fluid are the main clinical features. Therefore, fasting for three weeks is necessary postoperatively. A viable musculocutaneous flap should close the mediastinal space and separate the esophagus from other important structures in the mediastinum, such as the trachea and great vessels. The two cases of esophageal anastomotic leakage we described were suffered from very small pits, which difficult to detect by esophagogram and gastroscopy. Applying methylene blue could be helpful in diagnosis. After drainage and antibiotics, the anastomotic leak healed well through the ongoing inflammation and fibrosis, and no other serious complications were observed.
Tracheal anastomosis complications are another concern for us. Although none of the patients have experienced at the current stage, we are concerned that tracheal complications may occur after the cases increased. We believe that a tension-free airway anastomosis and well blood supply protection can reduce this risk, however, we have only three cases here, and a larger sample sizes and longer-term studies are needed to observe and verify the safety.
The decision to utilize a myocutaneous flap to repair the TEF rather than primary suture is based on the following reasons. Unlike congenital TEF, acquired TEF that result from endotracheal intubation or previous surgical procedures undergo severe inflammatory reactions at the fistula site and surrounding tissues, leading to the formation of numerous scars. This can result in two outcomes: (I) the esophagus may be narrowed, and direct repair could cause more severe stenosis, and (II) repairing the esophagus on the scar tissue increases the risk of postoperative fistula recurrence. If the scars are excised, it may worsen the stenosis after-repair. The use of the flap to repair the esophagus not only perfectly addresses the issue of esophageal stenosis but also facilitates rapid adhesion formation, effectively addressing the potential complications of perforation or leak, and reducing postoperative mortality. We have previously attempted primary suture, but the clinical outcomes were not satisfactory.
Department of otolaryngology-head and neck surgery
Repair using pedicled flaps is an effective technique based on related studies (10,17). The TAPF is considered a workhorse flap by many otolaryngology-head and neck surgeons because of its versatility and reliability. A relevant anatomic study has demonstrated that a constant thoracoacromial artery perforator is present in the septum between the clavicular and sternocostal heads of the pectoralis major muscle. The territory of the TAPF extended up to the fourth intercostal space inferiorly, and the mean length of the vascular pedicle was 7.1 cm (18). The sufficient pedicle length makes it a suitable donor-site area for esophageal reconstruction through a midsternal incision, and the wide territory of the flap allows for effective widening of the stenotic esophagus. It is also suitable for reconstruction of the esophagus in the narrow mediastinal space because the TAPF offers a thinner flap than the latissimus dorsi flap and pectoralis major flap, which cause the esophagus and trachea to undergo milder external pressure after reconstruction. A longer vascular pedicle provides more flexible transposition without chest deformity compared with an internal mammary artery flap.
Vascular anastomosis is not required when using a pedicled musculocutaneous flap to repair the defect compared with free flaps, so the operation could be simpler and easier to apply.
Several issues on the treatment of these patients were further discussed as follows
Question 1: The midsternal incision repair procedure provided a wide and accessible operation field. However, the trachea must be traversed during this procedure. Are the benefits of severing the trachea greater than the risks?
Expert opinion 1: Dr. Benoit Jacques Bibas
Anterior opening of the trachea to access the esophagus is a traditional method of TEF primary repair. It has the advantage of having a very good exposure of the defect and avoids dissection of the lateral portion of the trachea, which could induce lesion to the laryngeal nerves. Furthermore, many cases will have associated tracheal stenosis that will need resection. If a good viable tension-free airway anastomosis is performed, I would not expect any risks with this approach.
Expert opinion 2: Dr. Hei-Yu Matthew Chen
There are two parts to this question; first part being whether sternotomy approach to repair of a TEF confers additional benefit versus a more widely adopted approach via a right thoracotomy. The second question being whether the risk of severing the trachea is justified.
Regarding the first part of the question, sternotomy with tracheal division would allow excellent access to the anteriorly located esophageal defect. Undoubtedly this would allow parachuting of the TAPF as shown by the authors for a secure repair. However, a more widely adopted approach via right thoracotomy may also provide satisfactory exposure to the esophageal defect albeit being less accessible via the technique described by the author. In patient requiring lower tracheal surgery, right thoracotomy or even right video-assisted thoracoscopic surgery (VATS) allow for full mobilization of the trachea to achieve a tension free anastomosis. Although the author’s point of there being more adhesions in this type of patient is well recognized, it is reasonable to believe that the same type of mobilization required for tracheal resection can be similarly used to mobilize the trachea adequate for patch repair of the esophagus. I would like to propose a challenge to authors whether the same patch repair may be possible via right thoracotomy and even right VATS approach.
Secondly if the decision for tracheal division is made as described by the authors, adequate dissection and mobilization of the trachea is necessary to achieve a tension free anastomosis, especially since patients with TEF would likely develop more severe adhesions as described by the authors.
Expert opinion 3: Dr. Takuya Nagashima
The risk of complications due to tracheal transection depends on the tension of the anastomotic site, blood supply, presence of comorbidities such as diabetes, and poor nutritional intake. However, when tracheal transection is treated adequately, the risk of complications is low. Considering the benefit of obtaining a far better surgical field, the risk is acceptable.
Expert opinion 4: Dr. Camilla Poggi
Several experiences of tracheal resection and reconstruction have been published with good long-term results (19,20). Regarding this approach, the impact of surgery is high but the rationale of TEF approach with fresh pedicled flap are so important that the benefits seem be balanced by the risk. This procedure has several aspects to be considered when surgeons plan the operation. In my opinion, when you choose this flap, the reason why the standard muscle flap (anterior neck muscles) is excluded must be strong, the transection of innominate vein should be done if the procedure is not otherwise possible (TEF position) and sternotomy as well. I cannot make any specific comment on the skin transposition because I do not have experience with this flap.
Question 2: The sufficient territory and vascular pedicle length of the TAPF make it a suitable donor-site area for upper or middle esophageal reconstruction. Do we have more alternative flaps?
Expert opinion 1: Dr. Benoit Jacques Bibas
The TAPF is a suitable donor-site area for upper or middle esophageal reconstruction. Nonetheless, in our experience we did not need flaps for esophageal reconstruction. All our cases were performed with separation and direct suture of the esophagus. With this approach, oral feeding may be resumed in 5–7 days and most patients are discharged in about 10 days, if no complications arise. The exception would obviously be a very large defect that would need a more complex reconstruction. Then we would proceed with a TAPF. But as I said, in our series, it was not necessary.
Expert opinion 2: Dr. Hei-Yu Matthew Chen
An alternative option for a myocutaneous flap would be that of the use of an internal mammary artery perforator flap. As the authors suggested, it is limited by its cosmetic concerns. Furthermore, usage of an internal mammary artery perforator has the added concern of theoretically reducing the blood supply to the sternum and thus predisposing to deep sternal wound infections and sternal dehiscence.
On the other hand, an intercostal muscle flap represents a familiar option for thoracic surgeons and can be similarly interposed between the esophagus and trachea as described in multiple case reports. The flap can also be readily harvested via a right thoracotomy or even a right VATS approach and can be mobilized to cover the defect from a mid to low TEF.
Expert opinion 3: Dr. Takuya Nagashima
There are several options for the flaps for esophageal reconstruction. As the author mentioned in the discussion, a TAPF seems to be better in these cases.
Expert opinion 4: Dr. Camilla Poggi
The sufficient territory and the vascular supply in terms of arterial length of this TAPF make it a suitable donor-site area for upper or middle esophageal reconstruction although some other flaps have been described more specifically and more extensively. The most common flaps for upper TEF are the anterior and lateral neck muscles (20-22). The outcomes of standard procedures are clear and repeated over time by different Authors so, even though the described option is a very fashionable alternative procedure, the feasibility of those more standard procedures must be excluded before performing more impactful and demanding surgical options.
Question 3: Unlike tracheal resection and anastomosis for primary tracheal tumor treatment, we found that adhesions between the trachea and the esophagus are more severe in TEF cases, and a defect in the tracheal membrane makes it virtually impossible to achieve tension-free tracheal repair after the trachea is mobilized. Is there another effective tracheal anastomosis solution?
Expert opinion 1: Dr. Benoit Jacques Bibas
Indeed, TEF cases have more adhesions. Probably due to the intense inflammatory reaction that occurs during the TEF process. Moreover, the fistula dissection will often lead to a somewhat large defect in the posterior wall of the trachea. So, it may be challenging to achieve a tension-free anastomosis. But I would not say that it is virtually impossible. It requires meticulous dissection of adhesions in order to properly perform the usual tracheal release maneuvers.
If the TEF repair is associated with a long-segment tracheal resection, a tension-free anastomosis may be difficult to achieve. In this situation, some options are available. A simple one is to perform a tracheostomy below the anastomosis. This reduces the pressure at the airway and protects the anastomosis from any minor leaks that may occur.
Another option would be to perform a supra-hyoid release. It releases the tension at the anastomosis but, on the other hand, often leads to dysphagia. So, I would be cautious to use it in elderly patients or during TEF repair.
Expert opinion 2: Dr. Hei-Yu Matthew Chen
Generally speaking, resection of up to 5 tracheal rings is considered acceptable for primary tracheal anastomosis. If tension is still a significant issue after mobilization of the trachea, it is reasonable to proceed to further mobilize 1–2 cm of both main bronchi to aid further mobilization of the distal trachea superiorly. This issue echoes back to what was posed in question 1 as to whether the risk of tracheal division and anastomosis is justified. If a tension-free tracheal anastomosis cannot be confidently achieved, then this approach should not be adopted. Furthermore, mobilization of the distal trachea and bilateral main bronchi would be impossible with a sternotomy approach due to obstruction by the great vessels, again posing as a disadvantage of this approach.
Expert opinion 3: Dr. Takuya Nagashima
At first, the tracheal release maneuvers should be tried as much as possible; however, the membrane part should be dissected in a minimal area, to avoid loss of blood supply. The coverage of the anastomotic site is also important to minimize damage from fistulas. Fat tissue is one of the effective and convenient tissues for coverage, and it can separate the anastomotic site from the surrounding organs. The effectiveness of fat tissue for a bronchial stump was shown by several papers. The thymus and anterior mediastinal fat, which are resected to expose the trachea, could have been used in the reported cases.
Expert opinion 4: Dr. Camilla Poggi
This is more than true. Considering the principles of tracheal anastomosis in which tension and blood supply play the maximum in terms of expected outcome, and I would say tension is perhaps more important than blood supply, one possible solution is to perform release techniques. The chances of releasing are many and go from suprahyoid release maneuver through a cervical incision (23) and/or hilar and pericardial release, even bilateral (24). In this specific setting of TEF, the surgeons must remind that the procedure is almost a “one-shoot” option. A patient undergoing a such demanding procedure ending up with anastomosis tension is heading to failure. Timing and plan are key factors.
Conclusions
Repair of TEFs using TAPF through a midsternal incision approach provided a good exposure, and the procedure is not affected by scars or adhesions from previous operations, which simplifies the procedure and results in fewer complications in above cases. The TAPF has a constant thoracoacromial artery perforator and is easy to harvest; moreover, a sufficient length of the vascular pedicle provides a wide range for transfers. From our experience, although the incidence of anastomotic leak did not decrease significantly comparing with existing solutions, the patients did not encounter any serious systemic complications either. The anastomotic leak could eventually be cured with conservative treatment and has so far not had any deaths. Therefore, it is suitable for TEF repair in both the neck and the chest. We presume that this method is suitable not only for the repair of TEFs but also for benign esophageal strictures located in the upper segment of the esophagus. However, only 3 patients were included in this study, and more details must be verified by a larger sample size.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1128/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1128/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1128/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent form is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kang H, Yi KS, Kim SH, et al. Multidisciplinary team approach on tracheoesophageal fistula in a patient with home ventilator. J Thorac Dis 2022;14:4143-9. [Crossref] [PubMed]
- Leong CK, Foo AZX, Goh KJ, et al. Airway interventions for tracheobronchial involvement in esophageal carcinoma: a retrospective cohort outcome study and algorithmic approach. J Thorac Dis 2022;14:2565-78. [Crossref] [PubMed]
- Boybeyi-Turer O, Soyer T. Tracheoesophageal fistula after esophageal atresia repair: recurrent, missed or acquired. Curr Chall Thorac Surg 2022;4:26. [Crossref]
- Smithers CJ, Hamilton TE, Manfredi MA, et al. Categorization and repair of recurrent and acquired tracheoesophageal fistulae occurring after esophageal atresia repair. J Pediatr Surg 2017;52:424-30. [Crossref] [PubMed]
- Shen KR, Allen MS, Cassivi SD, et al. Surgical management of acquired nonmalignant tracheoesophageal and bronchoesophageal fistulae. Ann Thorac Surg 2010;90:914-8; discussion 919. [Crossref] [PubMed]
- Yang G, Xian L, Zhao W, et al. Surgical treatment for acquired tracheoesophageal fistula complicated with tracheal stenosis using endoscopic liner cutter staplers: a case report. Curr Chall Thorac Surg 2021;3:41. [Crossref]
- Cal S, Arslan S, Okur MH, et al. Analysis of mortality and long-term outcomes of pediatric patients with tracheoesophageal fistula/esophageal atresia. Ann Ital Chir 2023;94:231-9. [PubMed]
- Lal DR, Gadepalli SK, Downard CD, et al. Perioperative management and outcomes of esophageal atresia and tracheoesophageal fistula. J Pediatr Surg 2017;52:1245-51. [Crossref] [PubMed]
- Acher CW, Ostlie DJ, Leys CM, et al. Long-Term Outcomes of Patients with Tracheoesophageal Fistula/Esophageal Atresia: Survey Results from Tracheoesophageal Fistula/Esophageal Atresia Online Communities. Eur J Pediatr Surg 2016;26:476-80. [PubMed]
- Rosskopfova P, Perentes JY, Schäfer M, et al. Repair of challenging non-malignant tracheo- or broncho-oesophageal fistulas by extrathoracic muscle flaps. Eur J Cardiothorac Surg 2017;51:844-51. [Crossref] [PubMed]
- Balakrishnan A, Tapias L, Wright CD, et al. Surgical Management of Post-Esophagectomy Tracheo-Bronchial-Esophageal Fistula. Ann Thorac Surg 2018;106:1640-6. [Crossref] [PubMed]
- Li Z, Cui J, Zhang YX, et al. Versatility of the thoracoacromial artery perforator flap in head and neck reconstruction. J Reconstr Microsurg 2014;30:497-503. [Crossref] [PubMed]
- Lambertz R, Hölscher AH, Bludau M, et al. Management of Tracheo- or Bronchoesophageal Fistula After Ivor-Lewis Esophagectomy. World J Surg 2016;40:1680-7. [Crossref] [PubMed]
- Yanagihara T, Ichimura H, Kobayashi K, et al. Successful Surgical Closure of an Esophagobronchial Fistula Caused by a Foreign Body in the Esophagus of a Female Octogenarian with a Delayed Diagnosis: A Case Report. Ann Thorac Cardiovasc Surg 2021;27:126-31. [Crossref] [PubMed]
- Debourdeau A, Gonzalez JM, Dutau H, et al. Endoscopic treatment of nonmalignant tracheoesophageal and bronchoesophageal fistula: results and prognostic factors for its success. Surg Endosc 2019;33:549-56. [Crossref] [PubMed]
- Gonzalez JM, Servajean C, Aider B, et al. Efficacy of the endoscopic management of postoperative fistulas of leakages after esophageal surgery for cancer: a retrospective series. Surg Endosc 2016;30:4895-903. [Crossref] [PubMed]
- Hammoudeh ZS, Gursel E, Baciewicz FA Jr. Split latissimus dorsi muscle flap repair of acquired, nonmalignant, intrathoracic tracheoesophageal and bronchoesophageal fistulas. Heart Lung Circ 2015;24:e75-8. [Crossref] [PubMed]
- Zhang YX, Yongjie H, Messmer C, et al. Thoracoacromial artery perforator flap: anatomical basis and clinical applications. Plast Reconstr Surg 2013;131:759e-70e. [Crossref] [PubMed]
- D'Andrilli A, Maurizi G, Andreetti C, et al. Long-term results of laryngotracheal resection for benign stenosis from a series of 109 consecutive patients. Eur J Cardiothorac Surg 2016;50:105-9. [Crossref] [PubMed]
- Amore D, Casazza D, Caterino U, et al. Post-Intubation Tracheoesophageal Fistula: Surgical Management by Complete Cervical Tracheal Transection. Ann Thorac Cardiovasc Surg 2022; Epub ahead of print. [Crossref] [PubMed]
- Kim SP, Lee J, Lee SK, et al. Surgical Treatment Outcomes of Acquired Benign Tracheoesophageal Fistula: A Literature Review. J Chest Surg 2021;54:206-13. [Crossref] [PubMed]
- Puma F, Vannucci J, Santoprete S, et al. Surgery and perioperative management for post-intubation tracheoesophageal fistula: case series analysis. J Thorac Dis 2017;9:278-86. [Crossref] [PubMed]
- Broussard B, Mathisen DJ. Tracheal release maneuvers. Ann Cardiothorac Surg 2018;7:293-8. [Crossref] [PubMed]
- Liu X, Dai J, Li J, et al. Video-assisted thoracoscopic hilar and pericardial release for long-segment tracheal resections. J Thorac Dis 2022;14:3061-5. [Crossref] [PubMed]