Video assisted thoracoscopic plication of the left hemidiaphragm in symptomatic eventration in adulthood
Introduction
Diaphragmatic eventration is a congenital developmental defect of the muscular portion of the diaphragm, which maintains its “unbroken continuity” and its normal attachments to the dorsolumbar spine, the lower ribs, and the sternum. It has been attributed to abnormal myoblast migration to the septum transversum and the pleuroperitoneal membrane. Diaphragmatic eventration is rare (incidence <0.05%), being more common in males. It can be unilateral or bilateral, but it usually involves the left hemidiaphragm. It can partial, localized to a part of the hemidiaphragm (anterior, posterolateral, medial) or complete affecting the whole hemidiaphragm. Macroscopically, the (affected portion of the) diaphragm is attenuated, abundant, membranous, without muscular appearance. Microscopically there is paucity or absence of muscular fibers and diffuse fibroelastic changes. Eventration results in diaphragmatic elevation and cephalad displacement of the underlying abdominal viscera (1-3).
Eventration is differentiated from hernias, because in the former the diaphragm maintains its anatomical continuity, although it may appear thin and membranous (even translucent). True eventration is differentiated from diaphragmatic paralysis, because in the former there is absence of muscular layer without lesion of the diaphragmatic enervation, while in the latter (which is more common) the diaphragmatic muscular layer exists (even though it may be atrophic) and diaphragmatic elevation results from abnormalities of the neuromuscular axis between the spinal cord and the diaphragm (usually from phrenic nerve palsy after iatrogenic or other trauma, compression, infectious or malignant disease) (1-3).
Depending on the extent and the degree of muscle layer deprivation there is absence or less effective caudal movement of the diaphragm during inspiration. This leads to reduction of lung volumes and impaired ventilation. The most pronounced symptoms in infants with eventration are respiratory distress, feeding difficulties, and failure to thrive. Most adult patients with diaphragmatic eventration remain asymptomatic, and the diagnosis is made incidentally after chest radiography. Among symptomatic patients, the most common symptom is dyspnoea. Orthopnoea may occur due to further cephalad diaphragmatic displacement when lying down. There may be mild hypoxemia, causing tachypnoea and respiratory alkalosis. Palpitations may occur due to mainly supraventricular arrhythmias. Gastrointestinal symptoms may also be present, including epigastric discomfort or pain, heartburn, bloating, belching, constipation, and weight loss or inability to gain weight (mainly due to inappropriate food intake). Increased intraabdominal pressure (such as in strenuous exercise, obesity or pregnancy) may cause exaggeration of symptoms (1-5). Diaphragmatic rupture, gastric rupture, acute or chronic gastric or splenic flexure volvulus may rarely occur (6-10).
There is indication for surgical intervention only in the presence of symptoms (2-4). The established surgical treatment is diaphragmatic plication that can be achieved by various techniques and through various approaches: transthoracic or transabdominal, open, closed, or minimally invasive (2-4). Robotic repair has been reported in diaphragmatic anomalies (including eventration) in infants (11). In this study we describe a simple 3-port video assisted thoracoscopic plication of the left hemidiaphragm, reinforced by a bovine pericardial patch, employed in a symptomatic adult patient with left diaphragmatic eventration.
Operative technique
General anaesthesia with a double lumen tube was introduced. A nasogastric tube was inserted for complete gastric drainage. The patient was positioned in a right lateral position. When stomach drainage was deemed complete, the surgical table was tilted head up to facilitate descent of the abdominal viscera. The left lung was deflated before port insertion.
A video assisted thoracoscopic surgery (VATS) was performed for left hemidiahpragm plication. An oblique plication line was created starting anteromedially toward the cardiophrenic angle and progressing posterolaterally toward the costophrenic recess, using non absorbable interrupted sutures (Figure 1).
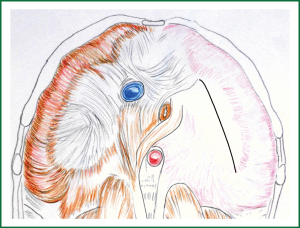 Figure 1. Desired plication line of the eventrated left hemidiaphragm (The diaphragm is viewed from the abdomen). The plication line is directed from anterolaterally to posteromedially, roughly coinciding or being parallel to the long axis of the left leaflet of the diaphragmatic central tendon. To achieve this final plication line, sutures are placed on each side of it, to approximate the corresponding parts of the diaphragm. (In reality the left leaflet of the central tendon is not well differentiated, due to partial or complete absence of the muscular portions of the hemidiaphragm. In the described case there was complete absence of the musclular layer, and thus there were no landmarks differentiating the left leaflet of the central tendon to the (amuscular) periphery. Nevertheless, the desired final plication line coincides with the “noetic” (i.e., perceived with the mind, virtual), long axis of the left leaflet of the central tendon of an anatomically normal left hemidiaphragm).
Figure 1. Desired plication line of the eventrated left hemidiaphragm (The diaphragm is viewed from the abdomen). The plication line is directed from anterolaterally to posteromedially, roughly coinciding or being parallel to the long axis of the left leaflet of the diaphragmatic central tendon. To achieve this final plication line, sutures are placed on each side of it, to approximate the corresponding parts of the diaphragm. (In reality the left leaflet of the central tendon is not well differentiated, due to partial or complete absence of the muscular portions of the hemidiaphragm. In the described case there was complete absence of the musclular layer, and thus there were no landmarks differentiating the left leaflet of the central tendon to the (amuscular) periphery. Nevertheless, the desired final plication line coincides with the “noetic” (i.e., perceived with the mind, virtual), long axis of the left leaflet of the central tendon of an anatomically normal left hemidiaphragm).
Thoracoscopic ports
Tree thoracoscopic ports 2-3 cm long were employed: the camera was inserted through the 5th intercostal space at the mid-axillary line. Endoscopic forceps and the needle holder were inserted through the 6th intercostal space at the anterior axillary line and the 8th intercostal space at the posterior axillary line. Occasionally more than one surgical instrument was inserted through one port.
Thoracoscopic findings
The lung, the parietal pleura, the mediastinum the phrenic nerve and the diaphragm were examined to exclude undiagnosed pathological process. The pulmonary ligament was transected. The hemidiaphragm appeared thin, abundant, fully deprived of muscular layer, with a cephalad displacement, reaching up to 2 cm below the left lung hilum, and having normal attachments. The hemidiaphragm was free of adhesions and freely moveable.
Creation of the first posterior pleat (P1)
Endoscopic forceps were introduced through the anterior port to grasp the abundant diaphragm at its dome 2.5 cm laterally to the posterior end of the cardiophrenic angle, anterolaterally to the oesophageal hiatus and well behind the phrenic nerve. A nylon 2-0 interrupted U stitch buttressed with a Teflon felt pledget was passed through a generous bite at the top of the pleat. The pledget was left at the posterior aspect of the pleat, the sutures were knotted at the anterior aspect of the pleat, the threads and needles were maintained (Figures 2,3,4).
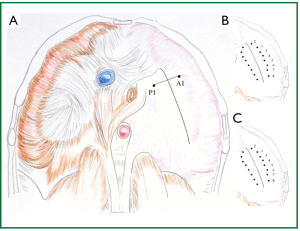 Figure 2. A. Placement of the first pair of sutures. A U stitch is placed at P1, laterally to the posterior end of the cardiophrenic angle, anterolaterally to the oesophageal hiatus. The sutures are tied, creating the first posterior pleat. Threads and suture needles are kept, and used for placement of a U stitch to A1, to create the first anterior pleat. B. After approximation of the A1 and P1, one more pair of similar sutures is placed anteromedially, towards the heart, and C. Six more pairs of sutures are placed posterolaterally. (The diaphragm is viewed from the abdomen).
Figure 2. A. Placement of the first pair of sutures. A U stitch is placed at P1, laterally to the posterior end of the cardiophrenic angle, anterolaterally to the oesophageal hiatus. The sutures are tied, creating the first posterior pleat. Threads and suture needles are kept, and used for placement of a U stitch to A1, to create the first anterior pleat. B. After approximation of the A1 and P1, one more pair of similar sutures is placed anteromedially, towards the heart, and C. Six more pairs of sutures are placed posterolaterally. (The diaphragm is viewed from the abdomen).
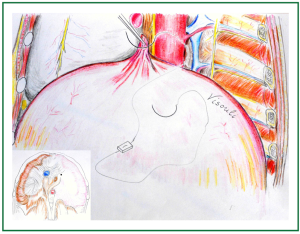 Figure 3. Creation of the first posterior pleat (P1): the thin and abundant diaphragm is grasped between forceps at its dome (2-3 cm peripherally the posterior end of the cardiophrenic angle, anterolaterally to the oesophageal hiatus). A U stitch is passed from the posterior to the anterior aspect of the pleat.
Figure 3. Creation of the first posterior pleat (P1): the thin and abundant diaphragm is grasped between forceps at its dome (2-3 cm peripherally the posterior end of the cardiophrenic angle, anterolaterally to the oesophageal hiatus). A U stitch is passed from the posterior to the anterior aspect of the pleat.
 Figure 4. Creation of the first posterior pleat (P1): the U stitch, buttressed with a Teflon felt pledget, is passed through the pleat, leaving the pledget at its posterior aspect. The sutures are tied and threads and needles are kept.
Figure 4. Creation of the first posterior pleat (P1): the U stitch, buttressed with a Teflon felt pledget, is passed through the pleat, leaving the pledget at its posterior aspect. The sutures are tied and threads and needles are kept.
Creation of the first anterior pleat (A1)
The diaphragm was grasped anteriorly, 2.5 cm posterolaterally to the anterior end of the cardiophrenic angle, anteriorly to the phrenic nerve. The sutures from the posterior pleat were passed though the top of the anterior pleat and a Teflon felt pledget that was placed at the anterior aspect of the pleat (Figures 5,6).
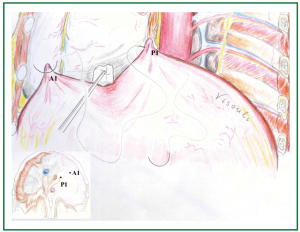 Figure 5. Creation of the first anterior pleat (A1): the diaphragm is grasped anteriorly (2-3 cm posterolaterally the anterior end of the cardiophrenic angle, anteriorly to the phrenic nerve) and a U stitch is passed, using the sutures of the first posterior pleat.
Figure 5. Creation of the first anterior pleat (A1): the diaphragm is grasped anteriorly (2-3 cm posterolaterally the anterior end of the cardiophrenic angle, anteriorly to the phrenic nerve) and a U stitch is passed, using the sutures of the first posterior pleat.
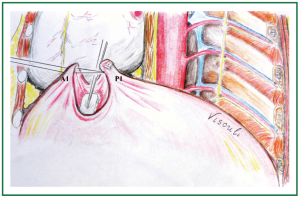 Figure 6. Approximation of the first posterior and anterior pleats (P1 and A1): after passing the stitches through the anterior pleat (A1) a Teflon felt pledget is also passed to be left at the anterior aspect of the anterior pleat. Gentle tension is applied to the sutures, while the diaphragmatic dome between the posterior and the anterior pleats is gently pushed towards the abdomen.
Figure 6. Approximation of the first posterior and anterior pleats (P1 and A1): after passing the stitches through the anterior pleat (A1) a Teflon felt pledget is also passed to be left at the anterior aspect of the anterior pleat. Gentle tension is applied to the sutures, while the diaphragmatic dome between the posterior and the anterior pleats is gently pushed towards the abdomen.
Approximation of the first posterior and anterior pleats (P1 and A1)
By applying gentle tension on the sutures, while the portion of the diaphragm between the sutures of the posterior and anterior pleats was gently pushed towards the abdomen (by a swab held in endoscopic forceps), the posterior and anterior pleats were approximated, and the sutures were knotted (over the anteriorly placed Teflon felt pledget of the anterior pleat), living the abundant diaphragmatic tissue caudal to the approximation site (Figures 6,7).
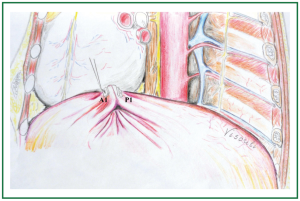 Figure 7. Approximation of the first posterior and anterior pleats (P1 and A1): when approximation of posterior and anterior pleats is complete the sutures are tied down. The abundant diaphragmatic dome in between the pleats has been inverted and invaginated underneath the approximated pleats.
Figure 7. Approximation of the first posterior and anterior pleats (P1 and A1): when approximation of posterior and anterior pleats is complete the sutures are tied down. The abundant diaphragmatic dome in between the pleats has been inverted and invaginated underneath the approximated pleats.
Progression of the approximation (plication) line centrally and peripherally
One pair of pledgeted sutures was placed in the same way anteromedially (towards the heart) and 6 more paired pledgeted interrupted sutures were similarly placed posterolaterally (towards the ribs), so that an oblique approximation (plication) line was created, starting anteromedially from the cardiophrenic angle (posteriorly to the phrenic nerve) and progressing posterolaterally to the costophrenic recess (the posterolateral diaphragmatic attachment to the ribs). The parallel U sutures were placed perpendicular to the desired final plication line, which crossed obliquely the eventrated diaphragmatic dome, coinciding with the anatomical position of the long axis of the left leaflet of the central tendon (Figures 1,2). Thus a posterior and an anterior fold were created and the abundant diaphragmatic dome was inverted, invaginated and left underneath, caudal to the approximation line of the buttressed sutures (i.e., the final plication line) (Figures 8,9).
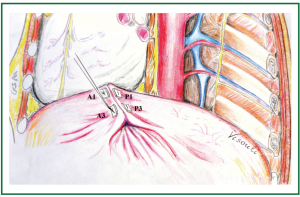 Figure 8. Approximation of the third posterior and anterior pleats (P3 and A3): in a similar manner, U stitches are passed through the third posterior and anterior pleats, progressing posterolaterally and leaving the abundant diaphragm underneath. (The second pair of sutures (P2 and A2, not shown) has already been placed and approximated, anteromedially to the approximated first pleats).
Figure 8. Approximation of the third posterior and anterior pleats (P3 and A3): in a similar manner, U stitches are passed through the third posterior and anterior pleats, progressing posterolaterally and leaving the abundant diaphragm underneath. (The second pair of sutures (P2 and A2, not shown) has already been placed and approximated, anteromedially to the approximated first pleats).
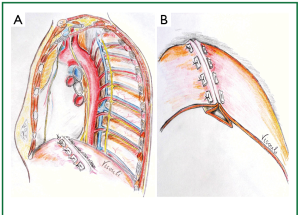 Figure 9. A. Progression of the plication line. B. By sequential approximation of the corresponding pairs of the posterior (P) and anterior (A) pleats, a posterior and anterior fold are created, lying underneath the plication line.
Figure 9. A. Progression of the plication line. B. By sequential approximation of the corresponding pairs of the posterior (P) and anterior (A) pleats, a posterior and anterior fold are created, lying underneath the plication line.
Reinforcement with pericardial patch
The plicated area was covered by a bovine pericardial patch (14 cm × 8 cm), placed with a continuous (over and over) running nylon suture (Figure 10).
Thus, by approximating the successive posterior and anterior pleats, a posterior and an anterior fold were created, approximated at a final plication line of an anteromedial to a posterolateral direction, with inversion and invagination of the abundant diaphragm caudal to the final plication line (Figures 8,9,11,12,13).
Finally a chest tube was inserted above the repaired diaphragm.
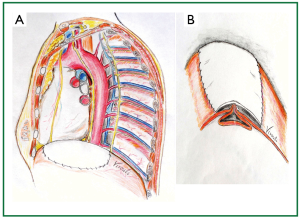 Figure 10. A. Patch reinforcement after completion of the plication. A 14 cm × 8 cm bovine pericardial patch was placed, with a continuous, running, non absorbable suture; B. Schematic representation of the plicated, and patch reinforced left hemidiaphragm.
Figure 10. A. Patch reinforcement after completion of the plication. A 14 cm × 8 cm bovine pericardial patch was placed, with a continuous, running, non absorbable suture; B. Schematic representation of the plicated, and patch reinforced left hemidiaphragm.
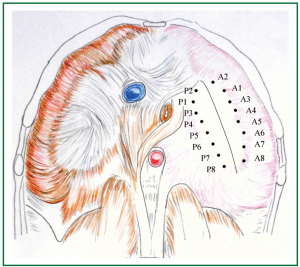 Figure 11. To achieve the desired final plication line, 8 pairs of sutures are placed on each side of it, to approximate the corresponding parts of the diaphragm. Suture placement results in creation of a pleat. Corresponding pleats are approximated. P1 and A1 are approximated first. P2 and A2 are approximated subsequently. P3 and A3 are then approximated, followed by approximation of the rest, progressing from antero-medially to postero-laterally. (The desired final plication line is perpendicular to the line segments P1A1, P2A2, …, P8A8, representing their segmental axis). The degree of plication depends on the distance between the pairs of posterior (P) and anterior (A) sutures (Pv - Αν) that create the posterior and anterior pleats. The longer the distance, the less the diaphragmatic surface left, thus the wider the degree of plication. Distance between posterior and anterior pleats that is too long leads to overcorrection, while a small distance leads to undercorrection. (In reality the distance between posterior (P) and anterior (A) sutures (and thus created pleats) is longer than that implied in the figure, because the sizes in the figure represent the normal sizes of the muscular periphery and the central tendon of the diaphragm (which are smaller than the sizes of the abundant eventrated diaphragm). The end result of approximation of the depicted posterior (P) and anterior (A) suture placement points (corresponding to the created posterior and anterior pleats) is diaphragmatic plication along the depicted line, that coincides with the anatomical position of the long axis of the left leaflet of the central tendon of a normal hemidiaphragm. The degree of plication and the eventual shape of the hemidiaphragm depend on the perceived by the surgeon “normal” shape, surface, position, and tension of the hemidiaphragm. We assume, though, that an estimation of the distance between the approximated parts can be made preoperatively, based on chest imaging, i.e., projection of diaphragms on chest x-ray, and preferably computed tomography, and 3 dimension reconstruction).
Figure 11. To achieve the desired final plication line, 8 pairs of sutures are placed on each side of it, to approximate the corresponding parts of the diaphragm. Suture placement results in creation of a pleat. Corresponding pleats are approximated. P1 and A1 are approximated first. P2 and A2 are approximated subsequently. P3 and A3 are then approximated, followed by approximation of the rest, progressing from antero-medially to postero-laterally. (The desired final plication line is perpendicular to the line segments P1A1, P2A2, …, P8A8, representing their segmental axis). The degree of plication depends on the distance between the pairs of posterior (P) and anterior (A) sutures (Pv - Αν) that create the posterior and anterior pleats. The longer the distance, the less the diaphragmatic surface left, thus the wider the degree of plication. Distance between posterior and anterior pleats that is too long leads to overcorrection, while a small distance leads to undercorrection. (In reality the distance between posterior (P) and anterior (A) sutures (and thus created pleats) is longer than that implied in the figure, because the sizes in the figure represent the normal sizes of the muscular periphery and the central tendon of the diaphragm (which are smaller than the sizes of the abundant eventrated diaphragm). The end result of approximation of the depicted posterior (P) and anterior (A) suture placement points (corresponding to the created posterior and anterior pleats) is diaphragmatic plication along the depicted line, that coincides with the anatomical position of the long axis of the left leaflet of the central tendon of a normal hemidiaphragm. The degree of plication and the eventual shape of the hemidiaphragm depend on the perceived by the surgeon “normal” shape, surface, position, and tension of the hemidiaphragm. We assume, though, that an estimation of the distance between the approximated parts can be made preoperatively, based on chest imaging, i.e., projection of diaphragms on chest x-ray, and preferably computed tomography, and 3 dimension reconstruction).
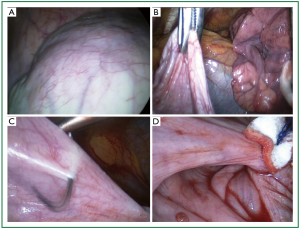 Figure 12. Intraoperative photographs. A. Thin and abundant left hemidiaphragm, fully deprived of muscular layer; B. The freely mobile dome grasped in endoscopic forceps; C. Placement of a Nylon 2-0 suture; D. Approximation of a posterior and anterior pleat. The abundant diaphragm is left underneath, caudal to the approximated pleats.
Figure 12. Intraoperative photographs. A. Thin and abundant left hemidiaphragm, fully deprived of muscular layer; B. The freely mobile dome grasped in endoscopic forceps; C. Placement of a Nylon 2-0 suture; D. Approximation of a posterior and anterior pleat. The abundant diaphragm is left underneath, caudal to the approximated pleats.
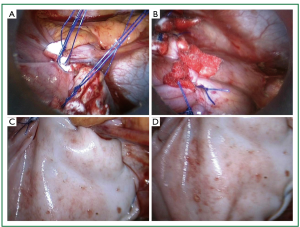 Figure 13. Intraoperative photographs. A. the first two pairs of sutures (A1P1 and A2P2) have been placed and approximated successively. B. Progression of the approximation line. C and D. Bovine pericardial patch reinforcement.
Figure 13. Intraoperative photographs. A. the first two pairs of sutures (A1P1 and A2P2) have been placed and approximated successively. B. Progression of the approximation line. C and D. Bovine pericardial patch reinforcement.
Patient characteristics and results
The described technique was applied in a 47-year old woman who presented with non-specific gastrointestinal symptoms (inadequate food intake due to dyspnoea and bloating after small meals, despite continuous gastrokinetic drug consumption), dyspnoea on exertion, and limited physical activity since childhood. She, surprisingly, had history of 2 full term pregnancies with normal delivery. She had no history of chest or neck trauma or operation, infectious or other systemic disease. A known, for at least 4 years, left hemidiaphragm elevation was confirmed on chest radiography. (Figures 14,15) Thoracic computed tomography showed a thin left hemidiaphragm, having normal attachments, and an elevated dome, with cephalad displacement of the underlying abdominal viscera (Figure 16). There was absence of lung or other thoracic mass, subdiaphragmatic abdominal mass, or lymph node enlargement (at the aortopulmonary window or elsewhere).
The recovery after the 3-port thoracoscopic plication was excellent. The patient was extubated in theatre, transferred to the ward without need of oxygen supplementation, and received liquid oral feedings 8 hours postoperatively. The early postoperative chest x-rays showed normal position of the left hemidiaphragm, without over- or under-correction (Figure 17). The patient underwent full mobilization and was discharged home on the 3rd postoperative day. She discontinued gastrokinetic medication (on the 8th postoperative day). One month postoperatively the Forced Expiratory Volume in 1 second (FEV1) was increased by 25% and the Forced Vital Capacity (FVC) by 11% in comparison to the preoperative values. One year postoperatively she remains well, reporting increased physical activity, and absence of dyspnoea and gastrointestinal symptoms. Weight gain, maintenance of normal anatomical position of the left hemidiaphragm on chest roentgenograms, and normal FEV1 and FVC were noted (Table 1).
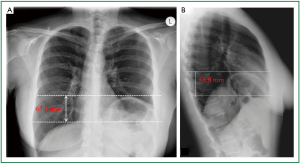 Figure 14. Preoperative chest radiography during inspiration. A. Distance between the parallel horizontal lines that are tangential to the apices of the projections of the hemidiaphragms, on anteroposterior chest x-ray: 65.8 mm; B. Distance between the parallel horizontal lines that are tangential to the apices of the projections of the hemidiaphragms on lateral chest x-ray: 58.9 mm.
Figure 14. Preoperative chest radiography during inspiration. A. Distance between the parallel horizontal lines that are tangential to the apices of the projections of the hemidiaphragms, on anteroposterior chest x-ray: 65.8 mm; B. Distance between the parallel horizontal lines that are tangential to the apices of the projections of the hemidiaphragms on lateral chest x-ray: 58.9 mm.
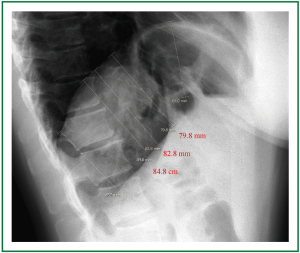 Figure 15. Preoperative lateral chest radiography. The distance between the parallel horizontal lines that are tangential to the apices of the projections of the hemidiaphragms in both anteroposterior and lateral chest x-rays is shorter than the distance of the (non horizontal) parallel lines tangential to various corresponding points of the eventrated and the normal hemidiaphragm. The 3 dimensional cephalad displacement and the resulting decrease in the lung volume may be underestimated by the anteroposterior diaphragmatic projection.
Figure 15. Preoperative lateral chest radiography. The distance between the parallel horizontal lines that are tangential to the apices of the projections of the hemidiaphragms in both anteroposterior and lateral chest x-rays is shorter than the distance of the (non horizontal) parallel lines tangential to various corresponding points of the eventrated and the normal hemidiaphragm. The 3 dimensional cephalad displacement and the resulting decrease in the lung volume may be underestimated by the anteroposterior diaphragmatic projection.
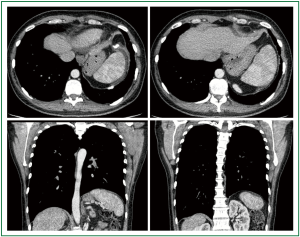 Figure 16. Preoperative thoracic computed tomography. Thin and elevated left hemidiaphragm with cephalad displacement of the underlying abdominal viscera.
Figure 16. Preoperative thoracic computed tomography. Thin and elevated left hemidiaphragm with cephalad displacement of the underlying abdominal viscera.
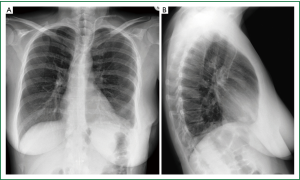 Figure 17. Chest radiography during inspiration, on the 3rd postoperative day. Normal anatomical position of the left hemidiaphragm, without overcorrection or undercorrection, on posteroanterior (A) and lateral (B) chest X-rays.
Figure 17. Chest radiography during inspiration, on the 3rd postoperative day. Normal anatomical position of the left hemidiaphragm, without overcorrection or undercorrection, on posteroanterior (A) and lateral (B) chest X-rays.
Full table
Discussion
Various approaches have been employed for diaphragmatic plication, including open transthoracic approaches (various thoracotomies, sternotomy, hemi clam shell incision), thoracoscopic approaches (various numbers, lengths, and location of ports), mimimally invasive approaches (small thoracotomies and thoracoscopically assisted mini thoracotomies), and open transabdominal, or laparoscopic approaches (1-4).
Various techniques of diaphragmatic plication have also been employed. The techniques applied for true diaphragmatic eventration and diaphragmatic paralysis are similar. All techniques aim to reduce the abundant diaphragmatic surface and lower the diaphragmatic dome (12). Various suturing methods have been used, including (buttressed or not) interrupted horizontal mattress sutures, multiple parallel U sutures, figure of eight sutures, continuous running sutures, and endostaplers. Various non absorbable but also absorbable sutures have been used.
According to early, and a few recent reports, excision of the abundant diaphragm was performed. In 1959, Christensen (1) reported oval radial excision and suturing in 2 or 3 layers by non absorbable material, through an open transthoracic approach. In 1970, Thomas (2) reported opening of the membranous portion of the diaphragm, freeing of the adhesions to the underlying abdominal viscera, resection of the excess membranous portion, and a two-layer overlapping approximation with nonabsorbable interrupted sutures, through an open transthoracic approach. In 2006, Di Giorgio et al. (13) reported dome incision and excision of a portion of the eventrated diaphragm (which was thin and transparent), followed by “dual-layer sandwich mesh repair”, through an open transthoracic approach. In 2009, Hori et al. (14) reported laparoscopic resection of an abundant eventrated hemidiaphragm using endostaplers.
In most recent reports though, diaphragmatic resection is not performed. The redundant part is not resected but is plicated by suturing. When folds are created they lie underneath the plication line. When a redundant central portion is left after suturing, it is usually invaginated underneath the plication sutures, but it can also be left cephalad to it and used to cover it (15).
When folds are created, the “classic” direction of plication is the posterior costo-phrenic angle to the cardio-phrenic angle (thus posterolateral to anteromedial) (12), but various other directions have been used, including oblique (anterolateral to posteromedial), transverse, or both transverse and anteroposterior (3,4,15).
Freeman et al. (16) reported plication to treat diaphragmatic paralysis, by placing 6-8 parallel U stitches, beginning medially and progressing laterally, usually through video assisted thoracoscopic surgery (VATS) but also through an open transthoracic approach. Leo et al. (12) described the “reefing the mainsail” plication technique to treat diaphragmatic paralysis, by creating an anterior transverse fold and anchoring it to the anterolateral arch of the 6th rib, using interrupted absorbable sutures. This technique can be employed through any open anterior approach (sternotomy, hemi clam shell).
Mouroux et al. (17,18) reported diaphragmatic plication to treat “eventration” (mainly due to phrenic nerve lesion after operation, trauma or infection), through a video assisted technique (performed through 2 ports and a 4 cm mini thoracotomy without rib spreading), creating a transverse fold, using 2 superposed back-and-forth continuous sutures. The first suture starts from the periphery and proceeds to the cardiophrenic angle, posteriorly to the phrenic nerve, invaginating the eventrated diaphragm and maintaining its excess inside the abdomen. The second suture stretches the dome at the desired tension. Groth et al. (3,4,19) reported a 4-port laparoscopic diaphragmatic plication to treat eventration or paralysis, starting with posteroanterior plication (beginning as far posteriorly as possible), followed by a transverse plication in a medial to lateral direction. They used non absorbable interrupted pledget reinforced U sutures, creating a T shaped plication suture line. Hwang et al. (20) reported a 4-port thoracoscopic oblique diaphragmatic plication to treat eventration, with a continuous running non absorbable suture, starting at the anterolateral and proceeding to the posteromedial costophrenic recess, invaginating the abundant diaphragm and progressively inverting it caudally. Kim et al. (21) reported a 3-port thoracoscopic plication to treat diaphragmatic paresis, using CO2 insufflations and interrupted figure of eight sutures, starting at the middle portion of the diaphragm and progressing posterolaterally and anteromedially. Moon et al. (22) reported thoracoscopic plication to treat diaphragmatic eventration, using knifeless endoscopic linear staplers. The redundant diaphragm was pulled, rolled and plicated without being resected; the procedure was repeated 5 times until desired diaphragmatic tension could be palpated.
Balci et al. (23) performed a retrospective comparison of diaphragmatic plication plus patch reinforcement to isolated diaphragmatic plication, through a minimal length lateral thoracotomy to treat diaphragmatic elevation (congenital or after surgery, disease or trauma). They concluded that patch reinforcement can protect from recurrence and herniation (observed in isolated plication). Different autologous or synthetic materials have been used for patch reinforcement of the repaired diaphragm (1,14).
We employed a video assisted thoracoscopic plication through 3 ports. Advantages of thoracoscopy are minimal surgical trauma, less postoperative pain, decreased risk of wound infection, potentially decreased postoperative morbidity and hospitalization, and better cosmetic result. Potential disadvantages include increased difficulty in estimation of sizes and analogies, in feeling the diaphragmatic tension during correction, but also increased technical difficulty due to decreased intrathoracic space, increased time of repair, and need for single contralateral lung ventilation. If the respiratory function and the general patient status allow safe video assisted thoracoscopic surgery (VATS) performance, the excess time spent for diaphragmatic repair is compensated by the time saved for opening and closure of a thoracotomy. Nevertheless, if an optimal and safe correction is not deemed feasible, conversion to a video assisted minithoracotomy seems reasonable. We have applied plication techniques similar to the described in the present study, to treat phrenic nerve paresis postcardiotomy, and diaphragmatic penetrations associated with catamenial pneumothorax, employing video assisted minithoracotomies.
We have chosen an oblique plication suture line starting anteromedially (at the cardiophrenic angle, posteriorly to the phrenic nerve), and proceeding posterolaterally to the costophrenic recess, because it appears “anatomically correct” coinciding or being parallel to the anatomical position of the long axis of left leaflet of the central diaphragmatic tendon. In case of existence of peripheral muscle layer the approximation results in maintenance of the three dimension muscle fiber orientation closer to normal. A vice versa direction of plication (starting posterolaterally and proceeding anteromedially) can also be applied, but the major anomaly appears at the dome and this is the point we have chosen to begin the plication. We believe that an oblique plication line coinciding with the anatomical position of the long axis of the left central tendon leaflet is the treatment of choice in complete or in partial eventration involving all parts of the diaphragm. In case of localized eventration involving one part of the diaphragm (if repair is required) a different plication line may be appropriate.
Incision of the diaphragm may be beneficial in case of adhesions to subdiaphragmatic viscera. When the diaphragm appears loose and easily mobilized we prefer to avoid its incision. We believe that in very thin diaphragms, fully deprived of muscular layer, suturing with hand sewn, interrupted, buttressed U stitches is a very good choice. Non absorbable sutures are probably preferable, particularly when non muscular parts are approximated. Bovine pericardial patch reinforcement was applied as an extra precaution to avoid recurrence or herniation.
The degree of the diaphragmatic surface decrease is a major aspect of the operation. The degree of plication (resulting in lowering of the diaphragmatic dome) depends on the distance between the approximated pleats (which defines the size of the final folds), and the length of the final plication suture line. In many reports the described aim is that the diaphragm is taut at completion. In our opinion, the aim should be that the repaired diaphragm is flattened at the dome, but not excessively taut, assuming its normal curvature to the periphery, avoiding overcorrection and undercorrection, as highlighted by Leo et al. (24).
There are no studies directly comparing the results of the various approaches and plication techniques (3). Moreover, in the majority of patients, plication is applied to treat paralysis rather than true eventration (the reason being that true eventration is rare in the adult). According to Groth et al. the choice of the plication technique depends on the expertise of the surgeon (4).
Graham et al. reported that the subjective and objective improvement achieved after open transthoracic plication in patients with unilateral diaphragmatic paralysis was sustained for 5 or more years (25) Mouroux et al. (18) reported that thoracoscopic plication (assisted by a 4 cm mini thoracotomy) mainly in patients with unilateral paralysis, had good long term results with complete relief of symptoms, absence of radiologic relapse at a mean follow-up of 64.4 +/- 46 months, and improvement in functional tests sustained for 5 years.
Groth et al. (2010) (3) reviewing the literature of “diaphragm plication for eventration or paralysis” concluded that: “Although the short-term outcomes after minimally invasive plication are promising, long-term results have yet to be published”. Gazala et al. (5) conducted a review [2012] including studies reporting plication for unilateral diaphragmatic paralysis, through open transthoracic or VATS approach. Thirteen observational studies, including a total of 161 patients were entered into their analysis that showed similar complication rate between the two groups, and lower mortality rate in the VATS group (0% in the VATS group vs. 4% in the thoracotomy group). The thoracoscopic approach appeared to have more advantages, regarding objective and subjective measures (including pulmonary functional tests, dyspnoea score, length of hospitalization, and postoperative complications). Nevertheless, due to the retrospective nature of the studies, patient selection (among other parameters) may have accounted for these differences.
Conclusively, we believe that VATS plication can be achieved with very good early and mid term results. Although long term results are not available, there is no reason to assume that an optimal thoracoscopic diaphragmatic plication, sustained for 1 to 5 year (as confirm by thoracic imaging and functional tests), will have late results inferior to those of an optimal plication performed through an open approach. Nevertheless longer- term follow-up is required to verify this assumption.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Christensen P., Eventration of the diaphragm. Thorax 959;311-9. [PubMed ]
- Thomas TV, Congenital eventration of the diaphragm. Ann Thorac Surg 1970;180-92. [PubMed ]
- Groth SS, Andrade RS, Diaphragm plication for eventration or paralysis: a review of the literature. Ann Thorac Surg 2010;S2146-50. [PubMed ]
- Groth SS, Andrade RS, Diaphragmatic eventration. Thorac Surg Clin 2009;511-9. [PubMed ]
- Gazala S, Hunt I, Bédard EL, Diaphragmatic plication offers functional improvement in dyspnoea and better pulmonary function with low morbidity.Interact Cardiovasc Thorac Surg 2012;505-8. [PubMed ]
- Morcillo-López I, Hidalgo-Mora JJ, Baamonde A, Gastric and diaphragmatic rupture in early pregnancy.Interact Cardiovasc Thorac Surg 2010;713-4. [PubMed ]
- Kanojia RP, Shanker R, Menon P, Eventration with diaphragm perforation leading to secondary diaphragmatic hernia and intestinal strangulation.Hernia 2010;531-3. [PubMed ]
- Oh A, Gulati G, Sherman ML, Bilateral eventration of the diaphragm with perforated gastric volvulus in an adolescent.J Pediatr Surg 2000;1824-6. [PubMed ]
- Goonetilleke G., Chronic volvulus of the stomach with eventration of the diaphragm.Ceylon Med J 1995;158-9. [PubMed ]
- Kim HS, Yoo JS, Han SJ, Chronic recurrent volvulus of the colonic splenic flexure associated with the eventration of left diaphragm.Korean J Gastroenterol 2007;37-40. [PubMed ]
- Slater BJ, Meehan JJ, Robotic repair of congenital diaphragmatic anomalies.J Laparoendosc Adv Surg Tech A 2009;S123-7. [PubMed ]
- Leo F, Girotti P, Tavecchio L, Anterior diaphragmatic plication in mediastinal surgery: the “reefing the mainsail” technique.Ann Thorac Surg 2010;2065-7. [PubMed ]
- Di Giorgio A, Cardini CL, Sammartino P, Dual-layer sandwich mesh repair in the treatment of major diaphragmatic eventration in an adult.J Thorac Cardiovasc Surg 2006;187-9. [PubMed ]
- Hori T, Masuda K, Taniguchi K, Transperitoneal laparoscopic surgery using endostaplers for adult unilateral diaphragmatic eventration.Surg Laparosc Endosc Percutan Tech 2009;e46-50. [PubMed ]
- Stolk J, Versteegh MI, Long-term effect of bilateral plication of the diaphragm.Chest 2000;786-9. [PubMed ]
- Freeman RK, Wozniak TC, Fitzgerald EB. Functional and physiologic results of video-assisted thoracoscopic diaphragm plication in adult patients with unilateral diaphragm paralysis. Ann Thorac Surg 2006;81:1853-7; discussion 1857.
- Mouroux J, Padovani B, Poirier NC, Technique for the repair of diaphragmatic eventration.Ann Thorac Surg 1996;905-7. [PubMed ]
- Mouroux J, Venissac N, Leo F, Surgical treatment of diaphragmatic eventration using video-assisted thoracic surgery: a prospective study.Ann Thorac Surg 2005;308-12. [PubMed ]
- Groth SS, Rueth NM, Kast T, Laparoscopic diaphragmatic plication for diaphragmatic paralysis and eventration: an objective evaluation of short-term and midterm results.J Thorac Cardiovasc Surg 2010;1452-6. [PubMed ]
- Hwang Z, Shin JS, Cho YH, A simple technique for the thoracoscopic plication of the diaphragm.Chest 2003;376-8. [PubMed ]
- Joo Hwang J, Kim KD. Thoracoscopic diaphragmatic plication using three 5 mm ports.Interact Cardiovasc Thorac Surg 2007;280-1. [PubMed ]
- Moon SW, Wang YP, Kim YW, Thoracoscopic plication of diaphragmatic eventration using endostaplers.Ann Thorac Surg 2000;299-300. [PubMed ]
- Balci AE, Ozyurtkan MO, Clinical and surgical specifications of adult unilateral diaphragmatic eventration according to their aetiology in 28 patients. Importance of using diaphragmatic patch and minimal thoracotomy incision.Eur J Cardiothorac Surg 2010;606-12. [PubMed ]
- Leo F, Venissac N, Morales F, Plication for diaphragmatic eventration: a simple technique, not a simple problem.Chest 2004;1170-author reply 1170-1. [PubMed ]
- Graham DR, Kaplan D, Evans CC, Diaphragmatic plication for unilateral diaphragmatic paralysis: a 10-year experience.Ann Thorac Surg 1990;248-51. [PubMed ]


