The sympathetic nervous system and catecholamines metabolism in obstructive sleep apnoea
Introduction
Obstructive sleep apnoea (OSA) is characterized by recurrent episodes of total (apnoea) o partial (hypopnea) upper airway collapse, which last ≥10 seconds and occur during sleep. They are usually associated with a reduction in blood oxygen saturation, respiratory effort, and arousals from sleep required to re-establish airway patency (1,2). The repetitive nature of these events during the night can lead to a prominent sleep fragmentation and to the development of typical signs and symptoms associated to OSA. These symptoms can be divided into those occurring during daytime (e.g., daytime sleepiness, morning headache, inability to concentrate, etc.) and those occurring during night time (e.g., snoring, choking or gasping during sleep, restless sleep, nocturia). Moreover, OSA is associated with cardiovascular (CV) complications, such as arterial hypertension (HT), pulmonary HT, arrhythmias, stroke, as well as with diabetes mellitus and metabolic syndrome (2). Multiple mechanisms have been contended to explain how OSA can increase CV risk and other morbidity factors, among which a key role can be played by the activation of the sympathetic nervous system (SNS), with related altered metabolism of catecholamines (CAs).
The relevance of OSA and the importance of a better understanding of its mechanisms is emphasized by the fact that sleep-related breathing disorders are common in middle-aged, overweight men and women. Because of this, due to the on-going obesity epidemic, the prevalence of OSA is steadily on the rise (3): in the 1990’s OSA involved 4% and 2% of middle-aged American men and women, respectively (4); current estimates are 10% and 3% of 30–49-year-old men and women, respectively (5,6). Therefore, aim of this review is to analyse the possible mechanisms by which OSA determines the dysregulation of autonomous nervous system (ANS), and thereby impact on CV risk.
Autonomous nervous system (ANS)
SNS and parasympathetic nervous system (PNS) interactively regulate visceral functions to maintain the body homeostatic milieu, and to enable reaction and adaptation to external and internal stressor stimuli (7,8). A very composite and intermingled mechanism operates at different levels, both centrally and peripherally, to coordinate the function of SNS and PNS. Under physiological conditions, a reciprocal activation of these two autonomic subsystems, the so-called “sympathovagal balance”, exists, which permits the activation of one branch, i.e., sympathetic outflow, alongside a withdrawal of the other, i.e., parasympathetic drive, and vice versa (9). However, it has been suggested that the co-activation of both is also possible, and could play a role in peculiar situations, such as chemoreceptor reflexes (i.e., during apnoea), exercise, and cold face immersion (10,11).
Catecholamines (CAs) production and metabolism
CAs are important neurotransmitters and hormones that play a fundamental role in the regulation of physiological processes, including one of most primordial reactions: the “fight or flight” response. Dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline) have positive inotropic, chronotropic, and dromotropic effects, leading to increased cardiac output, blood pressure (BP) and heart rate (HR). Moreover, they facilitate breathing by bronchodilation, and mobilize energy substrates from metabolic reserves by lipolysis and glycogenolysis.
The adrenal medulla and the post-ganglionic fibres of the SNS are the main sites of production, storage, and release of CAs. Synthesis of CAs relies on tyrosine, as a substrate, and involves activity of two enzymes, tyrosine hydroxylase (TH), and dopamine β-hydroxylase (DBH), which converts dopamine to norepinephrine (12) as showed in Figure 1.
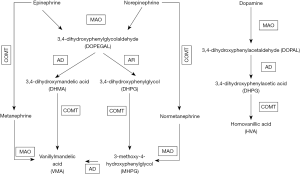
A key step for controlling norepinephrine and epinephrine effects entails their inactivation to the inactive metabolites, normetanephrine and metanephrine, respectively, operated by the enzyme catecol-O-methyl transferase (COMT). However, the latter also acts on others compounds with a catechol structure, as catecholestrogens and catechol-containing flavonoids. Hence, an altered activity of this enzyme can conceivably affect the bioactivity of all these substances.
COMT is coded by the COMT gene, located in the 22q11 region, which contains a non-synonymous single nucleotide polymorphism (rs4680G/A) that results in a valine (G allele) to methionine (A allele) mutation at position 158 (Val158Met) (13). This polymorphism was showed to imply different enzymatic activity: with the Val/Val carriers having a three-to-four-fold higher enzymatic activity than Met/Met carriers (14). Consequently, Met/Met women with a lower COMT activity showed higher oestrogen (estradiol) levels that could protect them from the development of HT and CV diseases (15). Notwithstanding this evidence, the role of altered COMT activity in OSA patients has never been investigated thus far, leaving unknown if it could affect the metabolism of CAs, and play a mechanistic role in HT and CV events.
How to measure the autonomic nervous system activity
Direct measurements
ANS activity can be measured directly or indirectly. Direct measurement of sympathetic nerve traffic in a given district can be accomplished with microneurography. This technique uses a tiny (about 100–200 µm in diameter, with a tip diameter of about 1 µm) tungsten microelectrode, with an epoxy resin-insulated shaft (impedance around 1–5 MΩ at 1 kHz), which is directly inserted in human peripheral myelinated and unmyelinated efferent and afferent fibres of muscle and skin nerves. The recording of efferent discharges in post-ganglionic sympathetic C efferent fibres innervating muscles and skin (muscle sympathetic nerve activity, MSNA, and skin sympathetic nerve activity, SSNA) provides information on the neural control of autonomic effector organs, including blood vessels and sweat glands. Accordingly, sympathetic microneurography represents a useful tool to investigate neural function and dysfunctions that involved BP control and body temperature regulation (16).
Moreover, the MSNA recording has been exploited to investigate changes in sympathetic neural traffic during sleep, and sleep-related events, such as sleep apnoea. These studies have shown that MSNA changes markedly during sleep: when the subject falls asleep MSNA decreases (17). Simultaneous sympathetic microneurography and polysomnographic recordings revealed that during supine sleep the reduction of burst rates of MSNA in the peroneal or tibial nerve was proportional to the depth of non-rapid eye movement (NREM) sleep. During rapid eye movement (REM) sleep the MSNA was almost as high as, or even higher than in the awake state (17). Of note, OSA patients with HT, who maintained a high MSNA level during NREM sleep, showed markedly elevated baseline MSNA, and less suppressed MSNA as compared to normal subjects (18,19). Unfortunately, the invasive and technically demanding nature of this technique prevented its widespread and regular use even in most clinical research settings.
Indirect measurements
Blood and urine CAs levels and their metabolic products
In 1995, Dimsdale et al. by measuring the plasma levels and urinary excretion of CAs in 43 hypertensive and normotensive individuals, with and without sleep apnoea, reported, for the first time, that 24-hour urinary norepinephrine levels were higher in patients with OSA than in patients without OSA (58.2 vs. 40.2 ng, P<0.002), both during the day and at night (20). We are going to analyse this indirect measurment in detail below in the text.
Power spectral analysis of heart rate variability (HRV)
HRV analysis entails a non-invasive reliable tool to assess the sympathetic and parasympathetic modulation, in physiological and pathological conditions (8,9,21). HRV depends on assessment of rhythmic oscillations embedded in heart period and BP time series, which reflect the sympathetic and parasympathetic modulations of CV function (22). The simplest method for analysing HRV is the calculation of the time domain measures, which can be derived from the analysis of the total electrocardiographic recording, or from shorter (but not shorter than 5 minutes) segments of the recording period. The most used time domain variables are: AVNN (ms) that represents the average of NN intervals for period of interest and can convert to average HR of NN intervals; the standard deviation of NN intervals-SDNN (ms)-that reflects total HRV; the standard deviation of AVNN for 5 minutes intervals for period of interest-SDANN (ms)-reflects primarily circadian HRV; the SDNN Index (ms), the average of 5 minutes standard deviations of NN intervals, represents the average of short-term HRV and combined SNS and PNS influences (21). Another method entails the frequency domain measures derived from the power spectral density (PSD), which provide information on how power (i.e., variance) distributes as a function of frequency (Table 1).
Variables derived from HRV analysis include the power of the high-frequency (HF) range (0.15-0.4 Hz), which reflects the parasympathetic modulation of the HR as sympathetic stimulation of the sinus node is substantially attenuated at frequencies above 0.15 Hz. At variance, the power of the low-frequency (LF) range reflects both sympathetic and parasympathetic activation. Accordingly, the LF/HF ratio is an estimation of the sympatho-vagal balance: an increased ratio indicates an enhanced sympathetic activity and/or a reduction of vagal activity. The physiological explanation of the very low-frequency (VLF) component is much less defined; it is considered a marker of hormonal and humoral fluctuations. VLF reflects underlying periodicities in HR at frequencies of every 25 seconds to every 5 minutes (0.0033–0.04 Hz). It involves the underlying frequency of most sleep-disordered breathing and periodic limb movements (PLMs). Limited data suggest that VLF can be modulated by a join action of the renin-angiotensin-aldosterone system [since it was reduced by angiotensin converting enzyme (ACE) inhibition] and the PNS (since it was blocked by atropine), but not by the SNS since it was not affected by beta-blockade (22).
Peripheral arterial tonometry (PAT)
This technology involves a finger-mounted pneumo-optical sensor that measures continuously the arterial pulse wave volume without the confounding effect of venous pulsations, which are eliminated. Episodes of upper airway obstruction cause vasoconstriction of digital vascular beds due to activation of the SNS, which results in attenuation of the PAT signal (23-25).
Sleep physiology and sympathetic nervous system (SNS)
The sleep process is characterized by the activation of a number of cortical, subcortical and medullar neural circuits, which cooperate in controlling sleep alongside hormonal changes (i.e., melatonin and orexin), local factors such as adenosine accumulation, circadian variations (i.e., dark-light cycles) and other unknown factors (26). A key role in the physiology of sleep is played by the ANS, which modulates CV functions during the onset of sleep and the transition to different sleep stages (27). Sleep stages classification depends on the observation of electroencephalographic waves and patterns, electro-oculographic patterns, and mental or submental muscle tone, as recorded by a full polysomnography. According to the American Academy of Sleep Medicine, the following terminology was recommended to separate the stage wakefulness (W) from the other two main, different sleep stages: NREM stages: N1, N2, and N3, and REM sleep. During normal sleep these stages progress cyclically from N1 through REM, then begin again with stage N1. A complete sleep cycle takes an average of 80 to 110 minutes with approximately 5 sleep cycles occurring in a normal night. The switch from W-to-sleep and among the different sleep stages is not mono-directional (i.e., from NREM to REM, and vice versa). This mechanism could be associated with the occurrence of arousals (28,29). It is well known that sleep is involved in the regulation of function of peripheral organs. In healthy subjects, during sleep, there are physiological changes in ventilation, HR, and BP that are predominantly sleep-stage dependent, and are mediated by modifications in autonomic control (30).
NREM sleep
During NREM sleep stages, particularly during stage N3, there is a progressive reduction in central respiratory drive, minute ventilation, and arterial oxygen pressure (PaO2), accompanied by a rise in arterial carbon dioxide pressure (PaCO2). During this stage there is a simultaneous increase in parasympathetic tone alongside a decrease in sympathetic activity, with ensuing reduction in HR, BP and BP variability, stroke volume, and systemic vascular resistance. The lowering of HR seems to be mainly related to the increase in the parasympathetic activity, whereas the decrease in BP appears to be primarily related to a reduction in the sympathetic vasomotor tone (17). The physiological respiratory sinus arrhythmia observed during NREM sleep is due to a sinusoidal modulation of HR variation. During inspiration, in order to ensure adaptation to the increased venous return, HR accelerates, resulting in an increase in cardiac output; during expiration HR slows progressively (31).
REM sleep
During REM sleep the cardiac efferent vagal tone is suppressed (32), SNS is increased to levels similar to those in W stage with an increase of HR and BP. Atonia of the respiratory muscles, excluding the diaphragm, and a reduction in chemo-sensitivity both occur in REM stage, and the combination of these two factors results in an increase in PaCO2, and decrease in ventilation to levels lower than that seen in NREM sleep (17).
Arousals
Arousals from sleep are associated with abrupt increases in both respiratory and CV activity; the associated augmentation of ventilatory drive exceeds that expected for the given PaCO2 level (33). At the same time, there is an increase in sympathetic activity and a decrease in cardiac vagal activity exceeding normal W stage levels, causing significant surges in HR and BP (30).
Sympathetic nervous system (SNS) and obstructive sleep apnoea (OSA)
In combination with other co-factors, sympathetic and catecholaminergic alterations play a key role in the pathophysiology of the CV morbidity in adults with OSA. The repetitive episodes of complete or partial obstruction of the upper airway during sleep lead to several consequences in particular an increase in airway resistance and respiratory effort that may produce oxygen desaturation, hypercapnia, and central nervous system arousal to restore airflow. These different mechanisms are associated with alterations of the hemodynamic balance related to changes in the activity of ANS (26,34,35).
The physiological responses to obstructive apnoea are triggered mainly by the reduction of PaO2. During hypoxia, chemoreceptor activation (so called “oxygen-conserving reflex”) promotes hyperventilation to enhance oxygen delivery to blood. This is followed by an increase of SNS activity that mediates vasoconstriction to redistribute oxygenated blood flow to vital organs. At the same time PNS is activated with consequent bradycardia, in order to reduce myocardial oxygen demand (17,36-38). Once normal breathing resumes, venous return and cardiac output increases. But the increase of the latter occurs into a highly constricted peripheral vasculature, which determines an increase of BP at the end of each apnoea. When the enhanced sympatho-excitation is sustained over years, as is the case in OSA patients, this physiologic response becomes pathological and can be responsible of the “non-dipping” BP profile at night, which, by itself, has been associated with an increased CV risk (30).
The respiratory and autonomic changes that occur during each acute obstructive apnoea episode are summarized in Figure 2.
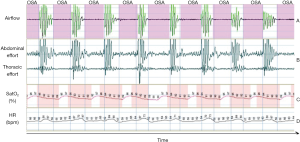
Other consequences of OSA are:
- Peripheral vasoconstriction, due to activation of the ANS, with consequent increase of peripheral resistance. This phenomenon persists for several seconds after ventilation resumption, due to the kinetics of norepinephrine uptake, release, and washout at the neurovascular junctions (44).
- Increase of the left ventricular transmural pressures, causing increased of the left ventricular afterload secondary to the augmented negative inspiratory intra-thoracic pressures generated against the occluded upper airway. These changes also increase venous return, and right ventricular pre-load, whilst OSA-induced hypoxia results in pulmonary vasoconstriction, leading to increases in right ventricular afterload. Collectively, right ventricular distension, impairment of left ventricular filling, and increased sympathetic activity increase myocardial oxygen demand in a setting in which apnoea-related hypoxia reduce tissue oxygen delivery (45).
As mentioned before, HRV represents an indirect measure of ANS activity. Sleep is the ideal condition to measure ANS activity, as it is not influenced by other conditions, such as physical activity and higher cortical function. The interaction between ANS and sleep is complex, bidirectional and regulated by several different factors. Changes in ANS regulation can profoundly affect sleep onset and sleep homeostasis; on the other hand, modifications of physiological sleep can influence the autonomic CV regulation. During sleep, autonomic cardiac control fluctuates between sympathetic and parasympathetic predominance, mainly depending on the transition to different stages (Figure 3). According to Somers et al. the CV system would be strongly affected by sleep stages in that W, NREM and REM sleep are related do a different haemodynamic status (17).
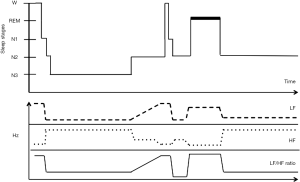
HR and BP variability are altered in patients with OSA, even in the absence of HT, heart failure, or other CV diseases. More importantly the degree of derangement in CV variability may be linked to the severity of OSA and, therefore, can represent a potential marker of sleep disorder breathing (18). Compared to controls, patients with OSA are characterized by a possible shift of the sympatho-vagal balance toward a sympathetic predominance and a vagal withdrawal, as shown by the increase of LF component and LF/HF, and the reduction of the HF component, either during night time or wake (30). This abnormal HRV pattern in patients with OSA has been confirmed in many studies although other authors could not replicate the same finding (Table 2). This is probably due to the different methods used to analyse HRV (fast Fourier transform, autoregressive method, coarse-graining spectral analysis), which limit the ability to compare adequately different studies.
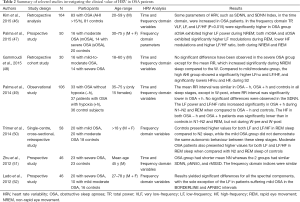
Full table
Other studies have showed the benefits of continuous positive airway pressure (CPAP) therapy on HRV (53-55), even after one night of treatment (56). CPAP is the gold standard therapy for moderate to severe OSA (57); long-term CPAP treatment can reduce the sympatho-vagal imbalance, mostly in patients with moderate and severe OSA, both during REM and NREM sleep (47).
Other alternative treatments for OSA have shown a similar beneficial effect on HRV modulation. Choi et al. (58) evaluated the effect of upper airway (UA) surgery on HRV using frequency domain analysis for patients who have had either successful (n=22) or unsuccessful (n=14) surgery. They described significant changes in the former group, and, in particular a significant reduction of VLF power and LF power. However, HF power and LF⁄HF ratio did not change significantly after UA surgery. Enhanced sympathetic activity in subjects with OSA has been observed even during W, in conditions of stationarity (normal breathing and normoxia). In a recent study, comparing patients with severe OSA (AHI ≥30), with and without heart failure, who were split into two subgroups according to the presence or absence of daytime sleepiness-measured through the Epworth Sleepiness Scale (ESS) questionnaire-subjects without excessive daytime sleepiness (EDS) had higher harmonic VLF power than patients with EDS. The ESS scores correlated inversely with VLF power in all subjects (r=−0.294, P=0.005) and in heart failure subjects (r=−0.468, P=0.016), which led the authors to conclude that in patients with heart failure, VLF harmonic power during sleep could be a more reliable index of sympathetic modulation of HR than the usual indices such as LF/HF and LF power. OSA appeared to be a stimulus potent enough to entrain sympathetic discharge in the VLF range of the apnoea-hypernea cycle (59).
Studies in humans have shown increased muscle sympathetic nerve activity (MSNA) under hypoxic conditions at simulated altitudes, which persisted even after removal of acute short-term exposure to intermittent hypoxia (60-63). Therefore, night time chronic intermittent hypoxia might contribute to the activation of the central nervous system and to the increase in peripheral chemosensitivity that are carried over into wakefulness, resulting in sustained daytime sympathetic overactivity (64).
The mechanisms involved in this phenomenon are:
- Sustained elevation in CAs levels with down regulation of vascular sympatho-adrenergic receptors: a reduced response to the intra-arterial infusion of α- and β-receptor agonists was observed in OSA patients when compared to normotensive subjects (65);
- Polymorphisms in α- and β-adrenergic receptor genes: the Arg389Gly polymorphism of the β1-adrenergic receptor has been associated with an increased risk of OSA development in hypertensive men. Furthermore, occurrence of HT increased in subjects with mild OSA with increasing number of Arg389 alleles. A lack of an association has been reported between polymorphisms of the β2-adrenergic receptor gene and OSA and between α- adrenergic receptor genes and OSA (66);
- Cardiac vagal dysfunction: cardiac vagal regulation is comprised of a tonic and a dynamic component, which can be assessed by spectral analysis of HRV (22);
- Baroreflex changes: the baroreflex sensitivity is reduced during sleep and W in OSA patients compared to control subjects (67). In patients with refractory HT and OSA a single night of CPAP treatment was found to be associated with an increase in baroreflex sensitivity and decrease in systolic BP (68).
Over the years some studies have shown the presence of elevated levels of CAs in both urine and plasma suggesting increased sympathetic activity in patients with OSA. Moreover treatment with CPAP and other therapies, such as tracheostomy, lowered CAs levels (45,69-73). Elmasry et al. showed that, in a population-based sample of hypertensive males (n=116), OSA was associated with significantly increased urinary excretion of normetanephrine and metanephrine, when compared to non-OSA subjects. This association was independent of major confounding factors (age, BMI and severity of HT) (74). More recently Kohler et al., in 102 males with moderate-to-severe OSA, randomised to therapeutic (n=51) or sub-therapeutic (n=51) CPAP treatment, showed that 4 weeks of therapeutic CPAP induced a significant reduction in urine normetanephrine excretion (179.7±80.1 to 132.7±46.5 mcmol·mol−1 creatinine) which did not occur in the sub-therapeutic group (75).
However, data from other studies and clinical trials have revealed a substantial variability regarding the effect of CPAP treatment on CAs levels and BP: some researchers reported a reduction in circulating CAs that extended into daytime and wakefulness (70,75), while others could not confirm a significant difference between treated and untreated OSA patients (76). Comondore et al. analysed 24-h urinary extraction of CAs, microalbumin, creatinine ratio, 24-h BP profile, and endothelial function markers in two groups of patients with AHI >15/h either treated with CPAP therapy or untreated. Patients treated with CPAP showed a trend to improvement of all these parameters, which, however, did not reach statistical significance (P>0.10) (77). These contrasting results can be explained by the fact that the therapeutic effect of CPAP on BP and sympathetic outflow is more prominent in hypertensive than in normotensive OSA patients, as showed by Heitmann et al. (78), who assessed the effect of CPAP therapy on norepinephrine kinetic. They found that in OSA patients CPAP treatment leads to a reduction in renal sympathetic neural activity by enhancing the renal clearance of norepinephrine rather than by attenuating norepinephrine synthesis rates. Kohler et al. have also showed that CPAP withdrawal was accompanied by elevation in urinary CAs (79). Thus, all these studies support the assumption that OSA patients are characterised by elevations in CAs levels that are attenuated by treatment.
Conclusions
OSA affects sympatho-vagal modulation, both during sleep and wakefulness. Although the underlying mechanisms are complex and involve both branches of the ANS, OSA-induced ANS changes may represent a key pathophysiologic link between OSA, HT and CV disease. Furthermore, in OSA patients with HT, CPAP treatment might reduce BP and CV events through a modulation of the ANS activity, but more studies are necessary to fully understand the mechanisms that cause CV damage in OSA patients and, most of all, whether measuring ANS imbalance can help understanding which OSA patients are more at high risk of developing arterial HT.
Acknowledgements
Funding: This study was supported by FORICA (The Foundation for advanced Research in Hypertension and Cardiovascular disease), the Società Italiana dell’Ipertensione Arteriosa and the University of Padua to GPR.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Academy of Sleep Medicine. The international classification of sleep disorders, revised Diagnostic and Coding Manual. Library of Congress Catalog No. 97-71405.
- Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008;118:1080-111. [PubMed]
- Steier J, Lunt A, Hart N, et al. Observational study of the effect of obesity on lung volumes. Thorax 2014;69:752-9. [PubMed]
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5. [PubMed]
- Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006-14. [PubMed]
- Garvey JF, Pengo MF, Drakatos P, et al. Epidemiological aspects of obstructive sleep apnea. J Thorac Dis 2015;7:920-9. [PubMed]
- Malliani A, Pagani M, Lombardi F, et al. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991;84:482-92. [PubMed]
- Montano N, Porta A, Cogliati C, et al. Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neurosci Biobehav Rev 2009;33:71-80. [PubMed]
- Malliani A, Pagani M, Montano N, et al. Sympathovagal balance: a reappraisal. Circulation 1998;98:2640-3. [PubMed]
- Malliani A, Montano N. Heart rate variability as a clinical tool. Ital Heart J 2002;3:439-45. [PubMed]
- Paton JF, Boscan P, Pickering AE, et al. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res Brain Res Rev 2005;49:555-65. [PubMed]
- Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 2004;56:331-49. [PubMed]
- Lotta T, Vidgren J, Tilgmann C, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 1995;34:4202-10. [PubMed]
- Creveling CR. The role of catechol-O-methyltransferase in the inactivation of catecholestrogen. Cell Mol Neurobiol 2003;23:289-91. [PubMed]
- Worda C, Sator MO, Schneeberger C, et al. Influence of the catechol-O-methyltransferase (COMT) codon 158 polymorphism on estrogen levels in women. Hum Reprod 2003;18:262-6. [PubMed]
- Mano T, Iwase S, Toma S. Microneurography as a tool in clinical neurophysiology to investigate peripheral neural traffic in humans. Clin Neurophysiol 2006;117:2357-84. [PubMed]
- Somers VK, Dyken ME, Mark AL, et al. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 1993;328:303-7. [PubMed]
- Narkiewicz K, Pesek CA, Kato M, et al. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension 1998;32:1039-43. [PubMed]
- Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995;96:1897-904. [PubMed]
- Dimsdale JE, Coy T, Ziegler MG, et al. The effect of sleep apnea on plasma and urinary catecholamines. Sleep 1995;18:377-81. [PubMed]
- Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93:1043-65. [PubMed]
- Stein PK, Pu Y. Heart rate variability, sleep and sleep disorders. Sleep Med Rev 2012;16:47-66. [PubMed]
- Pillar G, Bar A, Betito M, et al. An automatic ambulatory device for detection of AASM defined arousals from sleep: the WP100. Sleep Med 2003;4:207-12. [PubMed]
- Park CY, Hong JH, Lee JH, et al. Clinical usefulness of watch-PAT for assessing the surgical results of obstructive sleep apnea syndrome. J Clin Sleep Med 2014;10:43-7. [PubMed]
- Schnall RP, Shlitner A, Sheffy J, et al. Periodic, profound peripheral vasoconstriction—a new marker of obstructive sleep apnea. Sleep 1999;22:939-46. [PubMed]
- Saper CB, Cano G, Scammell TE. Homeostatic, circadian, and emotional regulation of sleep. J Comp Neurol 2005;493:92-8. [PubMed]
- Tobaldini E, Nobili L, Strada S, et al. Heart rate variability in normal and pathological sleep. Front Physiol 2013;4:294. [PubMed]
- Iber C, Ancoli-Israel S, Chesson A, et al, editors. American Academy of Sleep Medicine: The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, ed 1. Westchester: American Academy of Sleep Medicine, 2007.
- Saper CB, Fuller PM, Pedersen NP, et al. Sleep state switching. Neuron 2010;68:1023-42. [PubMed]
- Mansukhani MP, Kara T, Caples SM, et al. Chemoreflexes, sleep apnea, and sympathetic dysregulation. Curr Hypertens Rep 2014;16:476. [PubMed]
- Baccelli G, Guazzi M, Mancia G, et al. Neural and non-neural mechanisms influencing circulation during sleep. Nature 1969;223:184-5. [PubMed]
- Mancia G. Autonomic modulation of the cardiovascular system during sleep. N Engl J Med 1993;328:347-49. [PubMed]
- Horner RL, Rivera MP, Kozar LF, et al. The ventilatory response to arousal from sleep is not fully explained by differences in CO(2) levels between sleep and wakefulness. J Physiol 2001;534:881-90. [PubMed]
- Bonsignore MR, Romano S, Marrone O, et al. Different heart rate patterns in obstructive apneas during NREM sleep. Sleep 1997;20:1167-74. [PubMed]
- Tilkian AG, Guilleminault C, Schroeder JS, et al. Hemodynamics in sleep-induced apnea: studies during wakefulness and sleep. Ann Intern Med 1976;85:714-9. [PubMed]
- Abboud FM, Thames MD. Interaction of cardiovascular reflexes in circulatory control. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology: Peripheral Circulation and Organ Blood Flow. Bethesda, Maryland, USA: American Physiological Society, 1984:675-753.
- Somers VK, Dyken ME, Mark AL, et al. Parasympathetic hyperresponsiveness and bradyarrhythmias during apnea in hypertension. Clin Auton Res 1992;2:171-6. [PubMed]
- Abboud F, Kumar R. Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J Clin Invest 2014;124:1454-7. [PubMed]
- Garpestad E, Ringler J, Parker JA, et al. Sleep stage influences the hemodynamic response to obstructive apneas. Am J Respir Crit Care Med 1995;152:199-203. [PubMed]
- Richardson DW, Wasserman AJ, Patterson JL Jr. General and regional circulatory responses to change in blood pH and carbon dioxide tension. J Clin Invest 1961;40:31-43. [PubMed]
- Eckberg DL, Rea RF, Andersson OK, et al. Baroreflex modulation of sympathetic activity and sympathetic neurotransmitters in humans. Acta Physiol Scand 1988;133:221-31. [PubMed]
- Elsenbruch S, Harnish MJ, Orr WC. Heart rate variability during waking and sleep in healthy males and females. Sleep 1999;22:1067-71. [PubMed]
- Gilmartin GS, Tamisier R, Curley M, et al. Ventilatory, hemodynamic, sympathetic nervous system, and vascular reactivity changes after recurrent nocturnal sustained hypoxia in humans. Am J Physiol Heart Circ Physiol 2008;295:H778-85. [PubMed]
- Imadojemu VA, Gleeson K, Gray KS, et al. Obstructive apnea during sleep is associated with peripheral vasoconstriction. Am J Respir Crit Care Med 2002;165:61-6. [PubMed]
- Hakim F, Gozal D, Kheirandish-Gozal L. Sympathetic and catecholaminergic alterations in sleep apnoea with particular emphasis on children. Front Neurol 2012;3:7. [PubMed]
- Kim YS, Kim SY. Clinical Implication of Heart Rate Variability in Obstructive Sleep Apnea Syndrome Patients. J Craniofac Surg 2015;26:1592-5. [PubMed]
- Palma JA, Iriarte J, Fernandez S, et al. Long-term continuous positive airway pressure therapy improves cardiac autonomic tone during sleep in patients with obstructive sleep apnea. Clin Auton Res 2015;25:225-32. [PubMed]
- Gammoudi N, Ben Cheikh R, Saafi MA, et al. Cardiac autonomic control in the obstructive sleep apnea. Libyan J Med 2015;10:26989. [PubMed]
- Palma JA, Iriarte J, Fernandez S, et al. Characterizing the phenotypes of obstructive sleep apnea: clinical, sleep, and autonomic features of obstructive sleep apnea with and without hypoxia. Clin Neurophysiol 2014;125:1783-91. [PubMed]
- Trimer R, Mendes RG, Costa FS, et al. Is there a chronic sleep stage-dependent linear and nonlinear cardiac autonomic impairment in obstructive sleep apnea? Sleep Breath 2014;18:403-9. [PubMed]
- Zhu K, Chemla D, Roisman G, et al. Overnight heart rate variability in patients with obstructive sleep apnoea: a time and frequency domain study. Clin Exp Pharmacol Physiol 2012;39:901-8. [PubMed]
- Lado MJ, Méndez AJ, Rodríguez-Liñares L, et al. Nocturnal evolution of heart rate variability indices in sleep apnea. Comput Biol Med 2012;42:1179-85. [PubMed]
- Nelesen RA, Yu H, Ziegler MG, et al. Continuous positive airway pressure normalizes cardiac autonomic and hemodynamic responses to a laboratory stressor in apneic patients. Chest 2001;119:1092-101. [PubMed]
- Roche F, Court-Fortune I, Pichot V, et al. Reduced cardiac sympathetic autonomic tone after longterm nasal continuous positive airway pressure in obstructive sleep apnoea syndrome. Clin Physiol 1999;19:127-34. [PubMed]
- Khoo MC, Blasi A. Sleep-related changes in autonomic control in obstructive sleep apnea: a model-based perspective. Respir Physiol Neurobiol 2013;188:267-76. [PubMed]
- Kufoy E, Palma JA, Lopez J, et al. Changes in the heart rate variability in patients with obstructive sleep apnea and its response to acute CPAP treatment. PLoS One 2012;7:e33769. [PubMed]
- Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009;5:263-76. [PubMed]
- Choi JH, Yi JS, Lee SH, et al. Effect of upper airway surgery on heart rate variability in patients with obstructive sleep apnoea syndrome. J Sleep Res 2012;21:316-21. [PubMed]
- Taranto Montemurro L, Floras JS, Picton P, et al. Relationship of heart rate variability to sleepiness in patients with obstructive sleep apnea with and without heart failure. J Clin Sleep Med 2014;10:271-6. [PubMed]
- Saito M, Mano T, Iwase S, et al. Responses in muscle sympathetic activity to acute hypoxia in humans. J Appl Physiol (1985) 1988;65:1548-52. [PubMed]
- Morgan BJ. Acute and chronic cardiovascular responses to sleep disordered breathing. Sleep 1996;19:S206-9. [PubMed]
- Xie A, Skatrud JB, Crabtree DC, et al. Neurocirculatory consequences of intermittent asphyxia in humans. J Appl Physiol (1985) 2000;89:1333-9. [PubMed]
- Cutler MJ, Swift NM, Keller DM, et al. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol (1985) 2004;96:754-61. [PubMed]
- Friedman O, Logan AG. Sympathoadrenal mechanisms in the pathogenesis of sleep apnea- related hypertension. Curr Hypertens Rep 2009;11:212-6. [PubMed]
- Grote L, Kraiczi H, Hedner J. Reduced alpha- and beta(2)- adrenergic vascular response in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2000;162:1480-7. [PubMed]
- Bengtsson Boström K, Hedner J, Grote L, et al. Polymorphisms in alpha- and beta- adrenergic receptor genes, hypertension, and obstructive sleep apnea: the Skaraborg Sleep Study. Int J Hypertens 2010;2010:458410.
- Ryan S, Ward S, Heneghan C, et al. Predictors of decreased spontaneous baroreflex sensitivityin obstructive sleep apnea syndrome. Chest 2007;131:1100-7. [PubMed]
- Logan AG, Tkacova R, Perlikowski SM, et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J 2003;21:241-7. [PubMed]
- Fletcher EC, Miller J, Schaaf JW, et al. Urinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertension. Sleep 1987;10:35-44. [PubMed]
- Baruzzi A, Riva R, Cirignotta F, et al. Atrial natriuretic peptide and catecholamines in obstructive sleep apnea syndrome. Sleep 1991;14:83-6. [PubMed]
- Ziegler MG, Nelesen R, Mills P, et al. Sleep apnea, norepinephrine-release rate, and daytime hypertension. Sleep 1997;20:224-31. [PubMed]
- Minemura H, Akashiba T, Yamamoto H, et al. Acute effect sof nasal continuous positive airway pressure on 24-hour blood pressure and catecholamines in patients with obstructive sleepapnea. Intern Med 1998;37:1009-13. [PubMed]
- Sukegawa M, Noda A, Sugiura T, et al. Assessment of continuous positive airway pressure treatment in obstructive sleep apnea syndrome using 24-hour urinary catecholamines. Clin Cardiol 2005;28:519-22. [PubMed]
- Elmasry A, Lindberg E, Hedner J, et al. Obstructive sleep apnoea and urine catecholamines in hypertensive males: a population-based study. Eur Respir J 2002;19:511-7. [PubMed]
- Kohler M, Pepperell JC, Casadei B, et al. CPAP and measures of cardiovascular risk in males with OSAS. Eur Respir J 2008;32:1488-96. [PubMed]
- Bao X, Nelesen RA, Loredo JS, et al. Blood pressure variability in obstructive sleep apnea: role of sympathetic nervous activity and effect of continuous positive airway pressure. Blood Press Monit 2002;7:301-7. [PubMed]
- Comondore VR, Cheema R, Fox J, et al. The impact of CPAP on cardiovascular biomarkers in minimally symptomatic patients with obstructive sleep apnea: a pilot feasibility randomized crossover trial. Lung 2009;187:17-22. [PubMed]
- Heitmann J, Ehlenz K, Penzel T, et al. Sympathetic activity is reduced by nCPAP in hypertensive obstructive sleep apnoea patients. Eur Respir J 2004;23:255-62. [PubMed]
- Kohler M, Stoewhas AC, Ayers L, et al. Theeffects of CPAP therapy withdrawal in patients with obstructive sleep apnea: a randomised controlled trial. Am J Respir Crit Care Med 2011;184:1192-9. [PubMed]



