Incidence and risk factors of chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a major long-term complication of acute pulmonary embolism (PE) that carries considerable morbidity and mortality (1-4). CTEPH incidence after acute PE varies significantly. Previously, CTEPH was considered a rare event, occurring in 0.1-0.5% of patients who survived an acute PE (1). However, recent studies suggest that the incidence of CTEPH is considerably higher (0.4-8.8%) than previously published in these patients (5-13). The exact incidence rate remains to be determined, particularly in Asian populations.
Understanding the process of CTEPH development after acute PE and identifying the risk factors for CTEPH, will improve early detection and prophylaxis for patients at risk. Although numerous individual factors have been linked with the development of CTEPH, including idiopathic PE (5), previous venous thromboembolism (VTE) (5,13,14), larger perfusion defect (3), and elevated systolic pulmonary artery pressure (SPAP) at the time of acute PE (13,15), the effects of acute PE management and embolus dissolution on CTEPH development have not been well documented.
In this study, we determined the incidence of CTEPH and evaluated associated risk factors in a large series of Asian patients with a first episode of acute PE.
Materials and methods
Patients
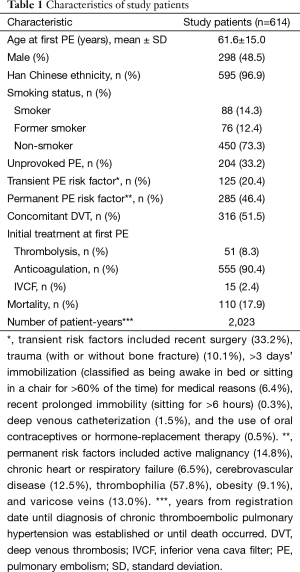
This consecutive cohort study was conducted in a national referral institute (Beijing Institute of Respiratory Medicine, Beijing Chao-Yang Hospital, Beijing, China) from January 1, 2006 to March 31, 2011. Written informed consent was obtained from all participants. The study protocol and consent procedure were approved by the ethics committee of Beijing Chao-Yang Hospital (ethics number: 2009-1).
Patients diagnosed with a first episode of acute PE were included. Patients with transient or permanent risk factors for PE were classified as having “provoked” PE. Transient risk factors included recent surgery, trauma (with or without bone fracture), >3 days’ immobilization (classified as being awake in bed or sitting in a chair for >60% of the time) for medical reasons, recent prolonged immobility (sitting for >6 hours), deep venous catheterization, pregnancy or being in the peripartum period, and the use of oral contraceptives or hormone-replacement therapy. Permanent risk factors included active malignancy, chronic heart or respiratory failure, cerebrovascular disease, thrombophilia, obesity, and varicose veins (16). PE occurring in the absence of risk factors was defined as “unprovoked” PE.
Patients with previously confirmed CTEPH or pulmonary hypertension (PH) at the time of acute PE diagnosis were excluded, as were those with any other medical condition that could have caused non-thromboembolic PH. Such conditions included severe chronic lung disease [forced expiratory volume in 1 second (FEV1) <60% of predicted value, FEV1/forced vital capacity (FVC) <60% of predicted, or total lung capacity <60% of predicted], left cardiac insufficiency (ejection fraction <30%), hematologic disorders (chronic hemolytic anemia or myeloproliferative disorders), systemic disorders (sarcoidosis, pulmonary Langerhans cell histiocytosis, or lymphangioleiomyomatosis), metabolic disorders (thyroid disorders, glycogen storage disease, or Gaucher disease), fibrosing mediastinitis, chronic renal failure, or any other pulmonary arterial hypertension-associated condition (human immunodeficiency virus, connective-tissue disease, portal hypertension, congenital heart diseases, or schistosomiasis).
Diagnosis and assessments
Acute PE was diagnosed by pulmonary angiography (PA), computed tomography (CT) PA (CTPA), or ventilation/perfusion (V/Q) scan (16-18). Deep venous thrombosis (DVT) was confirmed by ultrasonography of the leg veins or CT venography. The pulmonary embolism severity index (PESI) was evaluated upon admission (19,20). Severity stratification of acute PE was assessed using European Society of Cardiology (ESC) guidelines (8th edition) (16). All patients underwent echocardiography at the first episode of acute PE. The estimated SPAP at rest was equal to tricuspid regurgitation pressure gradient, calculated by the modified Bernoulli equation, plus the estimated right atrial pressure (19,21). The CT obstruction index was expressed as: [Σ(n∙d)/40]×100%, where n is the number of segmental branches arising in the pulmonary tree distal to the proximal thrombus (possible range, 1-20), and d is the degree of obstruction (possible range, 0-2) (22). Biomarkers of endothelial and fibrinolytic function assessed during the study were: D-dimer (normal value, 0-500 ng/mL), homocysteine (normal value, <15 µmol/L), plasminogen activator inhibitor-1 (normal range, 5-45 ng/mL), thrombomodulin (normal range, 3.10-5.82 ng/mL), tissue plasminogen activator (normal range, 1-12 ng/mL), protein C (normal range, 3.0-5.2 µg/mL), protein S (normal range, 19.0-26.8 µg/mL), and antithrombin III (AT-III) (normal range, 92.7-123.3%). Biomarkers were all measured by enzyme-linked immunosorbent assay (ELISA) method.
Treatment of PE
Hemodynamically stable acute PE was initially treated with either adjusted-dose unfractionated heparin or weight-based therapeutic doses of low molecular weight heparin. Hemodynamically unstable acute PE was initially treated with thrombolytic therapy, including streptokinase, urokinase, or recombinant tissue plasminogen activator. All patients (except for patients with cancer) started treatment with vitamin K antagonists within 1 week of diagnosis and continued for ≥6 months. Target international normalized ratio was 2.0-3.0. In patients with cancer, low molecular weight heparin was administered in the acute phase and continued over the first 3-6 months. Patients with unprovoked PE, recurrent VTE during follow-up, or at risk of recurrent VTE received prolonged anticoagulant treatment (>6 months) (16).
Follow-up and CTEPH diagnosis
Patients were followed up at 3, 6, 12, 18, and 24 months, and then yearly for up to 5 years. CTEPH was screened for and diagnosed by an independent expert panel. Patients with CTEPH were evaluated according to World Health Organization functional classification (19,23).
During outpatient follow-up, PH was assessed by an experienced technician using standardized transthoracic echocardiography. For patients with increasing SPAP, informed written consent was obtained before hemodynamic evaluation. Diagnosis of CTEPH was confirmed by right heart catheterization (RHC) in patients with multiple chronic or organized thromboembolic obstructions (from PA, V/Q scan, or CTPA) and defined as a mean pulmonary arterial pressure ≥25 mmHg, pulmonary capillary wedge pressure ≤15 mmHg, and pulmonary vascular resistance >2 Wood units (19,24,25). If written informed consent could not be obtained for hemodynamic evaluation, CTEPH was diagnosed according to time since acute PE >6 months, SPAP at rest >50 mmHg plus supportive results from V/Q scan and CTPA (2,10,19). In patients with CTEPH, CTPA shows the presence of eccentric thromboembolic material, subpleural densities, right ventricular enlargement, and a mosaic parenchymal pattern. Normal findings on V/Q scan effectively rule out CTEPH, whereas multiple bilateral perfusion defects suggest that CTEPH is a likely diagnosis (2). In the small number of patients who could not complete outpatient follow-up, clinical information was collected by telephone contact.
Statistical analysis
This study was considered a pilot and therefore no sample size calculations were required. The Kolmogorov-Smirnov test was used to assess the normality of continuous variables. Normally distributed data were expressed as mean values with standard deviation. Data that were not normally distributed were expressed as median and interquartile range. The cumulative incidence of CTEPH after acute PE was calculated using Kaplan-Meier methods. Cox regression analysis was performed to investigate risk factors for CTEPH. Multivariable regression analysis was used in a stepwise descending method from prognostic factors with a P value <0.1 in univariable analysis; results are given as hazard ratio (HR) and 95% confidence interval (CI). A P value <0.05 was considered statistically significant. SPSS version 15.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses.
Results
Patients
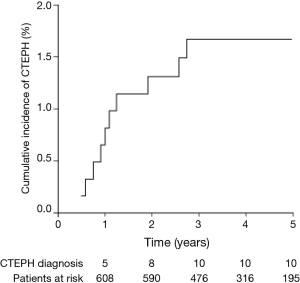
Acute PE was diagnosed in 695 patients. Of these, 614 patients (88.3%) were included in the study (Figure 1). Median follow-up was 3.3 years. Baseline characteristics are shown in Table 1. Of the 380 patients (61.9%) able to complete echocardiography, 27 met echocardiographic criteria for PH with SPAP >50 mmHg (Figure 1). CTEPH was diagnosed in nine of these patients. In 353 patients, estimated SPAP was low and diagnosis of PH could not be confirmed by echocardiography. Of these, one patient was diagnosed with CTEPH. No objective testing for PH was performed in the remaining 234 patients during follow-up. CTEPH was ruled out on clinical grounds in these patients as none of them had symptoms of unexplained dyspnea. Overall, 10 patients were diagnosed with CTEPH (four by RHC and PA; six by echocardiography-measured SPAP >50 mmHg plus V/Q scan and CTPA) (Figure 1).
Full table
During the study, 110 patients (17.9%) died, 20 patients (3.3%) from a direct result of acute (recurrent) PE, 62 (10.1%) from malignant disease, and 28 (4.6%) from other conditions.
Characteristics and outcomes of patients with CTEPH
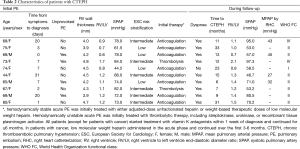
The characteristics of the ten patients with CTEPH are presented in Table 2. Nine patients underwent regular anticoagulant therapy before CTEPH developed. The remaining patient underwent anticoagulant therapy for 6 months and was diagnosed with CTEPH 17 months after drug discontinuation. The median time from acute PE to CTEPH was 12.5 months (interquartile range, 8.5-25 months). Despite being the recommended treatment, none of the patients diagnosed with CTEPH underwent pulmonary thromboendarterectomy (PEA), although four were assessed and deemed eligible for surgery. In some patients this was due to a preference for conservative management of their condition because of mild symptoms. Other reasons for patients not undergoing PEA were non-consent due to surgical risk or financial concerns resulting from personal circumstances and a lack of health insurance coverage. Clinical characteristics, treatments, and outcomes for patients are shown in Figure 2.
Full table
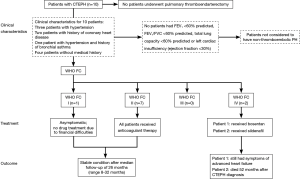
Incidence of CTEPH
The cumulative incidence of CTEPH after a first episode of acute PE was 0.8% (95% CI, 0.0-1.6%) at 1 year, 1.3% (95% CI, 0.3-2.3%) at 2 years, and 1.7% (95% CI, 0.7-2.7%) at 3 years. No further cases of CTEPH were seen after 3 years (Figure 3).
Risk factors for CTEPH
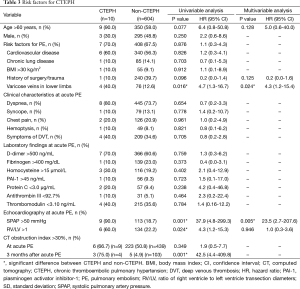
Patients older than 60 years were at higher risk of CTEPH (HR, 6.4; 95% CI, 0.8-50.8; P=0.077), but this was not significant in the multivariable analysis. A higher incidence of lower-limb vein varicosities were reported in patients with CTEPH vs. those without (HR, 4.7; 95% CI, 1.3-16.7; P=0.016), and multivariable analysis confirmed the association with CTEPH (HR, 4.3; 95% CI, 1.2-15.4; P=0.024) (Table 3).
Full table
The presence of SPAP >50 mmHg (HR, 37.9; 95% CI, 4.8-299.3; P=0.001) and ratio of right ventricle to left ventricle transection diameters >1 (HR, 4.3; 95% CI, 1.2-15.3; P=0.024) at acute PE were significantly higher in patients with CTEPH. In the multivariable model, elevated SPAP at acute PE was associated with CTEPH (HR, 23.5; 95% CI, 2.7-207.6; P=0.005) (Table 3).
CT obstruction index at acute PE did not differ between patients with and without CTEPH. Three months after acute PE, 107 patients (four with CTEPH and 103 without) were reassessed, at which time there was significant difference between patients with and without CTEPH (HR, 42.5; 95% CI, 4.4-409.8; P=0.001) (Table 3).
Based on ESC risk stratification, patients with intermediate-risk PE were at higher risk of CTEPH than those with low-risk PE (HR, 1.2; 95% CI, 1.0-1.4; P=0.030). High-risk PE, however, was associated with a lower risk of CTEPH than low-risk PE (HR, 0.7; 95% CI, 0.5-1.0; P=0.031). The same observation was made using the PESI assessment (HR, 0.9; 95% CI, 0.7-1.0; P=0.055).
Discussion
The cumulative incidence of CTEPH after a first episode of acute PE was 0.8% at 1 year, 1.3% at 2 years, and 1.7% at 3 years, with no further increase thereafter. A history of lower-limb varicose veins and an elevated SPAP (>50 mmHg) at acute PE were associated with increased risk of CTEPH. Additionally, residual emboli (CT obstruction index 3 months after acute PE) and intermediate-risk PE showed a tendency to increase the risk of CTEPH.
Data from a prospective study of 146 patients suggest that the majority of patients diagnosed with CTEPH have previously unidentified PH at the time of their initial acute PE (13). Therefore, a patient with a history of acute PE who is subsequently diagnosed with CTEPH may already have underlying CTEPH. In the study presented here, right ventricular wall thickness was measured as part of the echocardiographic assessment undertaken at the time of initial acute PE diagnosis. The right ventricular wall thicknesses of all 10 patients who were eventually diagnosed with CTEPH were below 5 mm at the time of their initial PE (Table 2), indicating that these patients had no overt signs of pre-existing chronic right ventricular dysfunction. We conclude, therefore, that it is unlikely that any of the 10 patients diagnosed with CTEPH had the condition prior to their initial diagnosis of acute PE.
Previous studies have shown an incidence of CTEPH after acute PE of 0.4-8.8% (Table 4). The substantial variation in incidence could be the result of the screened population, diagnostic methods and cut-off values, the duration of follow-up, and the size of the study population. In most previous studies (5,6,10,12), only patients with persistent dyspnea underwent echocardiography to screen for PH. In our study, all symptomatic and most asymptomatic patients were screened using echocardiography. This process identified one initially asymptomatic patient who developed CTEPH. While excluding asymptomatic patients may be more cost-effective, it may underestimate the incidence of CTEPH.
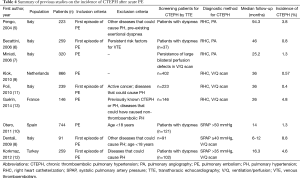
Full table
Current recommended best practice is for patients with suspected CTEPH to have the diagnosis confirmed by RHC and PA (3). However, in our study it was not always possible to carry these investigations out, due to either a lack of patient consent or the financial cost of the investigations, which was compounded by insufficient health insurance coverage. Given that an invasive assessment of hemodynamic function was not always possible, an alternative assessment of SPAP using echocardiography was utilized where necessary to diagnose PH.
SPAP cut-off values markedly affect the incidence of CTEPH. One study used a cut-off of >40 mmHg and found a particularly high incidence (8.8%) (8), while another used >35 mmHg and found a lower incidence (4.6%) (12). Two large screening studies assessed the reliability of several SPAP cut-offs using RHC as a reference. In the first, a cut-off of >38 mmHg had a false-positive rate of 45% (26). In the second, cut-offs of >33 and >36 mmHg had false-positive rates of 79% and 11%, respectively (27). In the current study, we found that a cut-off of 33-40 mmHg by echocardiography may overestimate the incidence of CTEPH. Conversely, a further study used >50 mmHg as the cut-off and found a CTEPH incidence of 1.3% (10), similar to that in studies using RHC to diagnose PH (5-7,9,11). Thus, CTEPH incidence based on SPAP >50 mmHg is more consistent with RHC findings. Indeed, the 2009 ESC guidelines recommend that SPAP >50 mmHg, with/without additional echocardiographic variables, is likely to indicate PH (evidence rating: IB) (19), and we used this cut-off to diagnose PH in patients who could not complete invasive procedures. The ESC guidelines also recommend SPAP 37-50 mmHg with/without additional echocardiographic variables as suggestive of PH (evidence rating: IIaC) (19). In our study, one patient with estimated SPAP <50 mmHg (46.7 mmHg) had unexplained dyspnea and was diagnosed with CTEPH by RHC and PA, suggesting that such patients may also develop CTEPH. The cumulative incidence of CTEPH reported here may, therefore, be an underestimate.
Numerous factors increase the risk of CTEPH after acute PE, such as younger or older age, recurrent VTE, idiopathic PE, history of malignancy, thyroid-replacement therapy, larger perfusion defect, more proximal PE, and SPAP >50 mmHg at acute PE (5,7,12-15). However, substantial differences in risk factors have been reported. In the present study, history of lower-limb varicose veins and SPAP >50 mmHg at acute PE were associated with increased CTEPH risk. Patients with lower-limb varicose veins might have asymptomatic DVT; left untreated, emboli gradually detach and block distal pulmonary arteries, eventually leading to CTEPH. In this study, most patients with SPAP >50 mmHg were in the intermediate- or high-risk PE category and were prone to CTEPH because of the large area of embolization. In patients with SPAP <50 mmHg, however, right ventricular dysfunction (24), and thus risk of CTEPH, was rarer. More attention should be paid to patients with varicose veins and high SPAP in outpatient services, and ultrasonography of leg veins and echocardiography should be repeated frequently.
Intermediate-risk PE (ESC classification) was associated with increased CTEPH risk. However, high-risk PE appeared to have a protective effect against CTEPH. This is likely because almost all patients with high-risk PE underwent thrombolytic treatment, resulting in more complete dissolution of emboli than in patients who initially received anticoagulant therapy. Of the ten patients with CTEPH, six had intermediate-risk PE, of whom four underwent initial anticoagulant therapy. We first assumed that initial anticoagulant therapy in patients with intermediate-risk PE would tend to increase the risk of CTEPH, and that thrombolytic treatment should be encouraged in these patients to reduce the development of CTEPH.
In a study of long-term outcomes of patients with acute PE undergoing surgical embolectomy, early postoperative mean SPAP (34.9±7.1 mmHg) was significantly lower than preoperative SPAP (44.9±5.7; P<0.001) (28). Mean SPAP at follow-up was 29.4±11.5 mmHg, a further significant decrease vs. the early post-operation value (P<0.001). Thus, pulmonary embolectomy can decrease pulmonary arterial pressure and maintain pressure normalization after surgery. In the current study, the CT obstruction index measured 3 months after acute pulmonary embolectomy was associated with an increased risk of CTEPH. We assume, therefore, that residual embolus after acute PE is a risk factor for CTEPH development.
The most significant limitations in our study were the fact that echocardiography was not able to be performed in all patients during the follow-up period, and that not all patients with increased SPAP underwent RHC and PA. As detailed above, an alternative definition of CTEPH was used in patients who were unable to undergo invasive hemodynamic assessment based on SPAP >50 mmHg as measured by echocardiography plus supportive results from a V/Q scan and CTPA (2,10,19). While this alternative diagnosis may be less accurate than RHC and PA, excluding patients who cannot complete the invasive examination would substantially underestimate the incidence of CTEPH after acute PE. The use of these alternative diagnostic criteria for CTEPH is a realistic reflection of current clinical practices in China, where many patients, either as a result of non-consent or economic disadvantage and a lack of health insurance coverage, will not be able to undergo procedures such as echocardiography, RHC, or PA. Another limitation of our study is related to the small number of patients with CTEPH. This could have caused bias in the univariable and multivariable analysis. However, our study was an exploratory study and we expect further research into the incidence and risk factors of CTEPH after acute PE in the future. In conclusion, we found that CTEPH is not a rare complication of acute PE in an Asian population. In this study population the cumulative incidence of CTEPH was ≥1.7% and developed within 3 years of acute PE. Physicians should be alert to patients with a history of lower-limb varicose veins, intermediate-risk PE with elevated SPAP in the acute phase, and residual emboli during follow-up. This will help identify early-stage CTEPH and maximize clinical resources for prevention, diagnosis, and treatment of CTEPH after acute PE.
Acknowledgements
The authors would like to thank the members of the independent adjudication committee (Yuanhua Yang, Yafeng Wu, Lei Zhang, and Zhanhong Ma) for the evaluation of VTE and PH in this study. Editorial assistance was provided by Adelphi Communications Ltd. (Bollington, UK), sponsored by Bayer Pharma AG.
Funding: This work was supported by China Key Research Projects of the 12th National Five-Year Development Plan (2011BAI11B17, 2012BAI05B01, 2012BAI05B02); and the Capital Health Research and Development Special Fund (No. 2011-4011-05).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fedullo PF, Auger WR, Kerr KM, et al. Chronic thromboembolic pulmonary hypertension. N Engl J Med 2001;345:1465-72. [PubMed]
- Piazza G, Goldhaber SZ. Chronic thromboembolic pulmonary hypertension. N Engl J Med 2011;364:351-60. [PubMed]
- Hoeper MM, Madani MM, Nakanishi N, et al. Chronic thromboembolic pulmonary hypertension. Lancet Respir Med 2014;2:573-82. [PubMed]
- Lang IM, Madani M. Update on chronic thromboembolic pulmonary hypertension. Circulation 2014;130:508-18. [PubMed]
- Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350:2257-64. [PubMed]
- Becattini C, Agnelli G, Pesavento R, et al. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest 2006;130:172-5. [PubMed]
- Miniati M, Monti S, Bottai M, et al. Survival and restoration of pulmonary perfusion in a long-term follow-up of patients after acute pulmonary embolism. Medicine (Baltimore) 2006;85:253-62. [PubMed]
- Dentali F, Donadini M, Gianni M, et al. Incidence of chronic pulmonary hypertension in patients with previous pulmonary embolism. Thromb Res 2009;124:256-8. [PubMed]
- Klok FA, van Kralingen KW, van Dijk AP, et al. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica 2010;95:970-5. [PubMed]
- Otero R, Oribe M, Ballaz A, et al. Echocardiographic assessment of pulmonary arterial pressure in the follow-up of patients with pulmonary embolism. Thromb Res 2011;127:303-8. [PubMed]
- Poli D, Grifoni E, Antonucci E, et al. Incidence of recurrent venous thromboembolism and of chronic thromboembolic pulmonary hypertension in patients after a first episode of pulmonary embolism. J Thromb Thrombolysis 2010;30:294-9. [PubMed]
- Korkmaz A, Ozlu T, Ozsu S, et al. Long-term outcomes in acute pulmonary thromboembolism: the incidence of chronic thromboembolic pulmonary hypertension and associated risk factors. Clin Appl Thromb Hemost 2012;18:281-8. [PubMed]
- Guérin L, Couturaud F, Parent F, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost 2014;112:598-605. [PubMed]
- Bonderman D, Wilkens H, Wakounig S, et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir J 2009;33:325-31. [PubMed]
- Ribeiro A, Lindmarker P, Johnsson H, et al. Pulmonary embolism: one-year follow-up with echocardiography doppler and five-year survival analysis. Circulation 1999;99:1325-30. [PubMed]
- Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2008;29:2276-315. [PubMed]
- Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet 2012;379:1835-46. [PubMed]
- Huisman MV, Klok FA. Diagnostic management of clinically suspected acute pulmonary embolism. J Thromb Haemost 2009;7 Suppl 1:312-7. [PubMed]
- Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT), et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009;34:1219-63. [PubMed]
- Aujesky D, Perrier A, Roy PM, et al. Validation of a clinical prognostic model to identify low-risk patients with pulmonary embolism. J Intern Med 2007;261:597-604. [PubMed]
- Lader E, Egan D, Hunsberger S, et al. The effect of digoxin on the quality of life in patients with heart failure. J Card Fail 2003;9:4-12. [PubMed]
- Qanadli SD, El Hajjam M, Vieillard-Baron A, et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol 2001;176:1415-20. [PubMed]
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004;43:40S-47S. [PubMed]
- Hoeper MM, Barberà JA, Channick RN, et al. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol 2009;54:S85-96. [PubMed]
- Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013;62:D92-9. [PubMed]
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005;52:3792-800. [PubMed]
- Sitbon O, Lascoux-Combe C, Delfraissy JF, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med 2008;177:108-13. [PubMed]
- Zarrabi K, Zolghadrasli A, Ali Ostovan M, et al. Residual pulmonary hypertension after retrograde pulmonary embolectomy: long-term follow-up of 30 patients with massive and submassive pulmonary embolism. Interact Cardiovasc Thorac Surg 2013;17:242-6. [PubMed]