Risk factors for distal stent graft-induced new entry following endovascular repair of type B aortic dissection
Introduction
Thoracic endovascular aortic repair (TEVAR) has been used for patients with type B aortic dissection (TBAD) since 1999 (1,2). Distal stent graft-induced new entry (DSINE), a new distal tear caused by the stent graft without natural disease progression or iatrogenic injury during endovascular manipulation, has been increasingly observed in recent years (3-14). To date, most studies on DSINE are focused on the mismatch between the true lumen and the distal diameter of the stent graft and limited by sample size, while studies on the risk factors for DSINE after TEVAR in large cohort of patients with TBAD are still lacking (6,9-11,14).
In a query of the patient database at Beijing Aortic Disease Center, we identified 39 cases of DSINE following initial TEVAR in 579 patients with TBAD (excluding connective tissue disorders) by February 2015. In this study, we seek to identify the risk factors of DSINE following TEVAR in patients with TBAD using multivariate analyses.
Methods
Patients
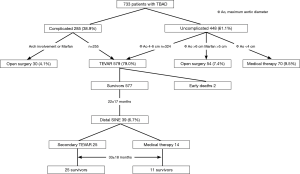
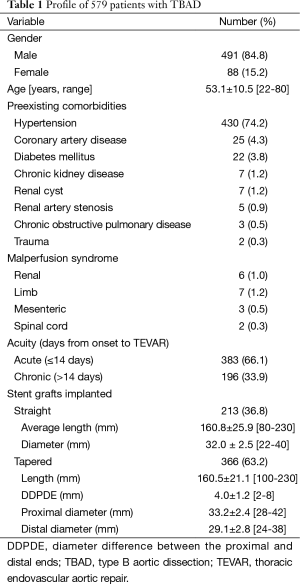
The ethics committee of Beijing Anzhen Hospital, Capital Medical University approved this retrospective study. Between January 2009 and January 2013, we treated 733 patients with TBAD, including 448 uncomplicated (61.1%) and 285 (38.9%) complicated cases. Open surgical repair was done in 84 patients (11.5%), including 30 complicated TBADs with aortic arch involvement (in 27 cases) or with connective tissue diseases (in 3 cases), and 54 uncomplicated TBADs with a maximum aortic diameter of ≥60 mm (in 46 cases) or with connective tissue disease and a maximum aortic diameter of ≥50 mm (in 8 cases). Seventy uncomplicated TBADs (9.5%) with a maximum aortic diameter of <40 mm were managed medically. The remaining 324 uncomplicated patients with a maximum aortic diameter of ≥40 mm and 255 complicated patients were managed with TEVAR, which comprises the study group of 579 cases (79.0%). Eleven patients (1.9%) with Marfan syndrome and other connective tissue disease were managed surgically. Figure 1 shows the breakdown of 733 patients based on their management strategies. Mean age was 53.1±10.5 years (range, 22-80 years) and 491 were male (84.8%). Hypertension was seen in 430 patients (74.2%). Based on the days from symptom onset, TBAD was acute in 383 patients (66.1%) and chronic in 196 (33.9%). The clinical profiles are listed in Table 1.
Full table
Inclusion and exclusion criteria
The indications for TEVAR in TBAD included complicated cases with unrelenting chest pain despite optimized medical therapy, uncontrollable hypertension, aortic growth rate >5 mm/6 months, malperfusion syndrome or imminent/contained rupture in 255 patients, and uncomplicated cases with a maximum aortic diameter ≥40 mm in 324 patients (Figure 1) (15). The exclusion criteria included type A (or retrograde type A) aortic dissection, ascending aortic aneurysms, unsuitable anatomy for TEVAR, Marfan syndrome or other connective tissue disorders, age of less than 18 years, pregnancy, systemic infection, and a landing zone >38 mm in diameter.
Devices and techniques
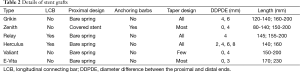
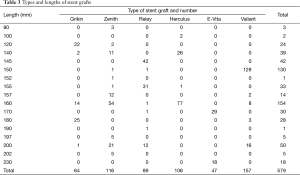
A total of 579 grafts were implanted in 579 patients. Six types of stent-graft devices were used (Table 2): Grikin (Grikin, Beijing, China) in 64 cases, Valiant (Medtronic, Minneapolis, MN, USA) in 157, Zenith TX2 proximal component (Cook, Bjaeverskov, Denmark) in 116, Relay (Bolton Medical, Sunrise, Fla) in 89, E-Vita (Jotec, Hechingen, Germany) in 47 and Hercules (MicroPort, Shanghai, China) in 106. All stent-grafts have a metal skeleton covered by polyester (Dacron). Some stent grafts used in earlier patients in this series were ≤145 mm in length, including the Grikin, Zenith TX2, Relay and Herculus.
Full table
TEVAR was performed in the catheter lab by a team of cardiologists, cardiac surgeons, radiologists and anesthesiologists. The endovascular procedure has been described in detail previously (8). We use a stent graft oversized by 10-15% in all patients.
Follow-up
Hospital survivors were clinically followed up with letters, emails, phone calls and by the local referring physician when needed. Computed tomographic angiography (CTA) was performed before discharge in all patients. After discharge, every patient was requested to have a repeat CTA at 1 month, 6 months and annually after the primary TEVAR.
Statistical analysis
Statistical analysis was performed using the SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). Variables were expressed as mean ± standard deviation or numbers (percentages). The differences among groups were compared with chi-square test or Fisher exact test as appropriate. Binary logistic regression was used to identify the risk factors for DSINE. Freedom from DSINE was estimated using the Kaplan-Meier method. All tests are 2-sided and a P value of <0.05 was considered statistically significant.
Results
Two patients (0.3%) died after the initial TEVAR. Spinal cord injury occurred in two (0.3%) patients, manifested as paraparesis. Stroke occurred in 3 patients (0.5%) and endoleak in 12 (2.1%). No DSINE occurred in the early postoperative period.
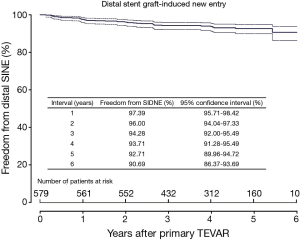
Clinical and radiological follow-up was complete in 100% (577/577). The duration of clinical follow-up was 47±16 months (range, 1-73 months). Late death occurred in six cases. DSINE occurred in 39 cases (6.7%) at 22±17 months after the initial TEVAR (range, 2-65 months). Freedom from DSINE was 92.7% (95% CI: 90.0-94.7%) at 5 years after the initial TEVAR (Figure 2). Other late events included retrograde type A aortic dissection in 6 cases (1.0%), endoleak in 13 (2.2%), subclavian steal syndrome in 3 (0.5%) and graft infection in 3 (0.5%). No patient sustained DSINE and retrograde type A dissection simultaneously.
Patients with DSINE were managed with re-TEVAR in 25 (4.3%, 25/579) and medically in 14 (2.4%, 14/579). At 33±18 months following DSINE, all patients managed with re-TEVAR (25/25) and 11 medically managed patients (11/14) survived (P=0.048).
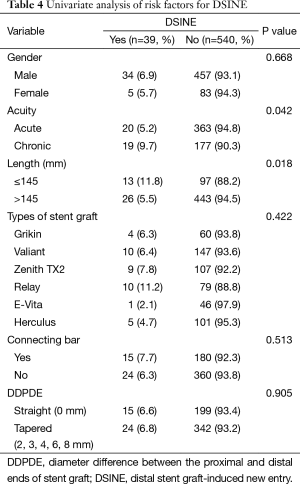
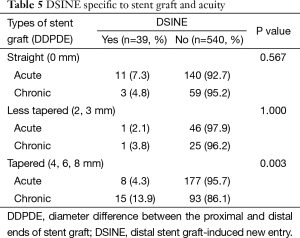
Univariate analysis identified two variables associated with DSINE: stent grafts of ≤145 mm in length (11.8%, 13/110 vs. 5.5%, 26/469; P=0.018) and TEVAR in the chronic phase (9.7%, 19/196 vs. 5.2%, 20/383; P=0.042). The incidence of DSINE did not decrease significantly in tapered stent grafts, nor did it differ with gender, types of stent grafts and connecting bar (Table 3). Using tapered stent grafts with a proximal end 4-8 mm larger than the distal end, TEVAR performed in the acute phase was associated with a significantly lower incidence of DSINE than those performed in the chronic phase (4.3%, 7/185 vs. 13.9%, 15/108; P=0.003) (Table 4).
Full table
Full table
In multivariate analysis, risk factors for DSINE were stent grafts of ≤145 mm in length [odds ratio (OR) 2.268; 95% CI: 1.121-4.587; P=0.023) and TEVAR done in the chronic phase (OR 1.935; 95% CI: 1.004-3.731; P=0.049) (Table 5).
Full table
Discussion
DSINE negatively impacts the long-term effects of TEVAR for patients with TBAD, with a reported incidence of 1.3-27% (3,4,6,8-14). In this series, the incidence of DSINE after TEVAR for TBAD was 6.7%. As one of the complications after TEVAR, DSINE may be more dangerous than TBAD itself. Usually, thrombosis fills with the false lumen around the stent graft and obliterates the initial outlet immediately after TEVAR. When DSINE occurs at the distal end of the stent graft where there is no outlet of the false lumen, a false aneurysm will develop eventually (Figure 3). Without timely reintervention, the risk of aortic rupture escalates and the natural prognosis is very poor. In this series, at 5 years after occurrence of DSINE, only 11 of 14 patients who were managed medically survived, while all patients managed with re-TEVAR (25/25) survived, which implies that re-TEVAR should be performed as early as possible. Dong and associates (6) also reported a mortality of 28.6% in patients with DSINE. In this context, identification of the risk factors for DSINE is very important for patients with TBAD undergoing TEVAR.
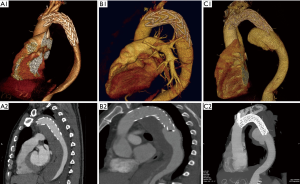
Endovascular stent grafting was first used for transluminal repair of abdominal aortic aneurysm in 1991 and thoracic aortic aneurysm in 1994 (16,17), and for repair of TBAD in 1999 (1,2). There was a significant difference in the incidence of DSINE between patients with descending aortic aneurysm and patients with TBAD in that the former has a normal distal landing zone while the latter has an abnormal distal landing zone with a dissected flap and narrowed true lumen (Figure 4A,B). In patients with TBAD, the taper ratio, defined as [(arch diameter—distal true lumen diameter)/arch diameter], was 13% in average in healthy individuals. Compared with normal controls, the taper ratio was about 20% larger in acute TBAD and 30% in chronic TBAD according to a previous study (18). Considering the mismatch between the true lumen and the distal diameter of stent graft, we speculated that the incidence of DSINE should be lower in patients who underwent primary TEVAR with tapered stent grafts than those with straight stent grafts in our series. However, no significant difference in the incidence of DSINE was found between the two groups (6.8% vs. 6.6%). So, other possible risk factors should also be suspected and examined (Table 4).
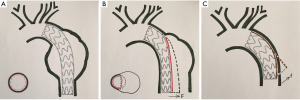
When anchored at the aortic arch, a self-expanding stent graft has the tendency to spring back to its initial straight status (5,6). With the leverage effect of the lesser curvature on a stent graft, this spring-back force on the distal end of the stent graft may play a role in the occurrence of DSINE. The present study showed that stent grafts less than 145 mm in length were associated with a higher incidence of DSINE. This may be the result of the leverage effect of the lesser curvature on a stent graft. After the proximal end of the stent graft is anchored in the aortic arch, the shorter the stent graft, the stronger the spring-back force at the distal end of the stent graft (Figure 4C). Therefore, reduction of this force may lead to better clinical outcomes by improving the conformability and elongating the stent graft so that stent graft stays in parallel with the straight part of the distal descending aorta.
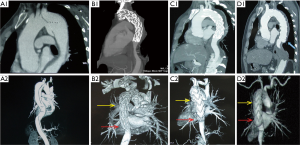
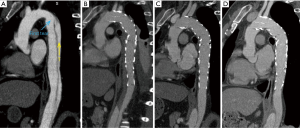
This study also identified TEVAR in the chronic phase as a risk factor for DSINE, which may be interpreted from a pathological perspective. Different from the acute phase, the mobility of the dissected intimal flap is decreased due to the degeneration and fibrosis in the chronic phase, and the dissected intimal flap could not return to its location prior to dissection (19). The narrowed true lumen and the fibrotic and thickened intimal flap could lead to compression of the distal part of stent-graft early after deployment of the stent-graft. In the long term, with the impact of blood flow, the intimal flap could not withstand the radial force exerted by the compressed distal part of the stent-graft. Eventually, the distal compressed stent graft fully expanded and SINE occurred at the distal margin of the stent-graft (Figure 5). Conversely, in acute TBADs, despite the narrowed true lumen and large primary entry tear, the distal part of the stent-graft can be fully opened because the mobile dissected flap can be easily molded back into its location prior to dissection with TEVAR (Figure 6).
This study did not find a significantly lower incidence of DSINE with tapered stent grafts, which might be associated with limited availability of taper ratios of current stent grafts. In this study, the average diameter difference of tapered stent grafts in this series was only 4.0±1.2 mm (average taper ratio: 12.2±3.6%), irrespective of the other risk factors, which might be insufficient to prevent DSINE in patients with acute TBAD and much more insufficient in chronic patients according to a previous study (18).
Although the incidence of DSINE did not differ significantly between stent grafts with and without connecting bars (Table 4) in this study, it is recommended that the connecting bar be removed to improve the flexibility of the stent graft, especially in the setting of a kinked aortic arch (20).
Based on the present study, it is our thought that the following measures may be helpful in preventing DSINE. First, a longer stent graft with better conformability should be chosen to minimize the leverage effect of the lesser curvature on the stent graft (Figure 6). Second, although there are the mismatches of diameter between the true lumen and distal diameter of stent graft in patients with both acute and chronic TBAD, tapered stent grafts with 4-8 mm in DDPDE should be effective to prevent DSINE in patients with acute TBAD given the better mobility of the dissected flaps. However, in patients with chronic TBAD, more tapered stent graft should be chosen based on the size of true lumen, we would add an extender of 120 mm in length and 10 mm in DDPDE when indicated due to the limited availability of tapered devices nowadays. Of course, the preemptive restrictive bare stent of proper size placed at the distal part of the intended stent graft is a good method to prevent DSINE (9,21,22), which can strength the distal anchoring zone and disperse both the radial force and the spring-back force at the distal end of the following stent graft. Most importantly, the mobility of the dissected flap should be assessed throughout the procedure. We would evaluate its movement or displacement with aortic angiogram at the mid-portion of descending thoracic aorta before stent graft implantation. Within 14 days of onset, apparent displacement can be found; the flap is very limited in movement beyond 14 days and seldom moves after 1 month of onset. After deployment of the first stent graft, if unfavorable mobility of the dissected flap is confirmed when the lower part of the stent graft is released incompletely and compressed, a more tapered extender may be helpful in preventing DSINE.
The present study was limited by its retrospective nature, the relatively small number of events, and the lack of longer follow-up after onset of DSINE. Despite these limitations, it has increased our understanding of DSINE and further studies are warranted.
Conclusions
Our results show that TEVAR performed during the acute phase and using stent grafts longer than 145 mm could decrease the incidence of DSINE in patients with TBAD. Tapered stent grafts with a proximal end 4-8 mm larger than distal end may be helpful in preventing DSINE after TEVAR performed in the acute phase than TEVAR performed in the chronic phase, due to the difference in mobility of the dissected flap. Repeat TEVAR should be performed expeditiously to improve the clinical prognosis of patients with DSINE.
Acknowledgements
Funding: This study was supported by the Special Research Fund for Public Welfare Industry of Health from National Health and Family Planning Commission of China (No. 201402009).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med 1999;340:1539-45. [PubMed]
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999;340:1546-52. [PubMed]
- Kiyotaka I, Shinichi S, Keiji U. Early and medium-term results of stent-graft treatment of DeBakey type III aortic dissection. J Cardiovasc Surg (Torino) 2006;47:651-7. [PubMed]
- Won JY, Suh SH, Ko HK, et al. Problems encountered during and after stent-graft treatment of aortic dissection. J Vasc Interv Radiol 2006;17:271-81. [PubMed]
- Riambau V, Guerrero F, Murillo I, et al. Stent grafting-related acute type B redissection. Vascular 2008;16:101-5. [PubMed]
- Dong Z, Fu W, Wang Y, et al. Stent graft-induced new entry after endovascular repair for Stanford type B aortic dissection. J Vasc Surg 2010;52:1450-7. [PubMed]
- Ricotta JJ 2nd. Invited commentary. Stent graft-induced new entry after endovascular repair for Stanford type B aortic dissection. J Vasc Surg 2010;52:1458. [PubMed]
- Xu SD, Huang FJ, Yang JF, et al. Early and midterm results of thoracic endovascular aortic repair of chronic type B aortic dissection. J Thorac Cardiovasc Surg 2010;139:1548-53. [PubMed]
- Feng J, Lu Q, Zhao Z, et al. Restrictive bare stent for prevention of stent graft-induced distal redissection after thoracic endovascular aortic repair for type B aortic dissection. J Vasc Surg 2013;57:44S-52S. [PubMed]
- Huang CY, Weng SH, Weng CF, et al. Factors predictive of distal stent graft-induced new entry after hybrid arch elephant trunk repair with stainless steel-based device in aortic dissection. J Thorac Cardiovasc Surg 2013;146:623-30. [PubMed]
- Weng SH, Weng CF, Chen WY, et al. Reintervention for distal stent graft-induced new entry after endovascular repair with a stainless steel-based device in aortic dissection. J Vasc Surg 2013;57:64-71. [PubMed]
- Andersen ND, Keenan JE, Ganapathi AM, et al. Current management and outcome of chronic type B aortic dissection: results with open and endovascular repair since the advent of thoracic endografting. Ann Cardiothorac Surg 2014;3:264-74. [PubMed]
- Hughes GC, Ganapathi AM, Keenan JE, et al. Thoracic endovascular aortic repair for chronic DeBakey IIIb aortic dissection. Ann Thorac Surg 2014;98:2092-7; discussion 2098. [PubMed]
- Jánosi RA, Tsagakis K, Bettin M, et al. Thoracic aortic aneurysm expansion due to late distal stent graft-induced new entry. Catheter Cardiovasc Interv 2015;85:E43-53. [PubMed]
- Dong ZH, Fu WG, Wang YQ, et al. Retrograde type A aortic dissection after endovascular stent graft placement for treatment of type B dissection. Circulation 2009;119:735-41. [PubMed]
- Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 1991;5:491-9. [PubMed]
- Dake MD, Miller DC, Semba CP, et al. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med 1994;331:1729-34. [PubMed]
- Xu SD, Huang FJ, Du JH, et al. A study of aortic dimension in type B aortic dissection. Interact Cardiovasc Thorac Surg 2008;7:244-8. [PubMed]
- Yang CP, Hsu CP, Chen WY, et al. Aortic remodeling after endovascular repair with stainless steel-based stent graft in acute and chronic type B aortic dissection. J Vasc Surg 2012;55:1600-10. [PubMed]
- Xu SD, Huang FJ, Yang JF, et al. Endovascular repair of acute type B aortic dissection: early and mid-term results. J Vasc Surg 2006;43:1090-5. [PubMed]
- Liu JF, Jiang WL, Lu HT, et al. Application of protective stents in endovascular repair of acute complicated Stanford type B aortic dissections. J Endovasc Ther 2013;20:210-8. [PubMed]
- He H, Yao K, Nie WP, et al. Modified Petticoat Technique with Pre-placement of a Distal Bare Stent Improves Early Aortic Remodeling after Complicated Acute Stanford Type B Aortic Dissection. Eur J Vasc Endovasc Surg 2015;50:450-9. [PubMed]


