Albert-Lembert versus hybrid-layered suture in hand sewn end-to-end cervical esophagogastric anastomosis after esophageal squamous cell carcinoma resection
Introduction
Thoracolaparoscopic esophagectomy (TLE) with 3-field lymphadenectomy is considered safe and feasible for patients with esophageal squamous cell carcinoma (ESCC) (1,2). Cervical esophagogastric anastomosis (CEGA) is preferred during esophagectomy because it eliminates the risk of postoperative mediastinitis due to intrathoracic anastomotic leaks, which are often associated with fatal consequences (3,4). Both of short-term complications include anastomotic leak and long-term complications include anastomotic stricture negatively impact patient’s quality of life after esophagectomy (5). Although the majority of CEGA leaks can be treated successfully with local wound care and adequate drainage (6), nearly half of the patients with anastomotic leak will develop an anastomotic stricture (7). When benign anastomotic stricture develops, recurrence of dysphagia defeats one of the main goals of esophagectomy, which is to relieve dysphagia and restore normal swallowing function (8). Thus, prevention of anastomotic leak and benign stricture after cervical anastomosis is essential for minimizing early morbidity and maximizing long-term functional results and quality of life (9).
The frequent and important problem of CEGA complications after esophagectomy has stimulated a variety of anastomotic techniques. CEGA could be performed either by a hand sewn or stapled anastomotic technique. Prospective randomized trials conducted by Hsu et al. (10) and Saluja et al. (7) reported that the incidence of anastomotic leak and stricture are comparable between hand sewn and stapled anastomosis. In addition, stapled technique is more expensive than hand sewn technique. Thus, hand sewn CEGA including end-to-end (ETE) and end-to-side (ETS) anastomosis has become the procedure of choice for a part of surgeons. A recent prospective randomized trial reported that ETS hand sewn anastomosis was associated with a higher anastomotic leak rate, while ETE hand sewn anastomosis was associated with a higher anastomotic stricture rate (11). To date, the ideal hand sewn CEGA technique remains elusive. In the past several years, a new ETE hand sewn CEGA technique named hybrid-layered suture was tried in our center. The aim of our study was to compare the rate of anastomotic leak and stricture between Albert-Lembert and hybrid-layered hand sewn ETE CEGA.
Patients and methods
Patients
This study was performed in the Xijing Hospital of Digestive Diseases affiliated to the Fourth Military Medical University. Between November 2010 and September 2012, 230 patients underwent esophagectomy with CEGA for ESCC were analyzed retrospectively. Preoperative staging was determined by endoscopic ultrasound (EUS) and enhanced abdominal-thoracic computed tomography (CT). This study was approved by the Ethics Committee of Xijing Hospital, and written informed consent was obtained from all patients before surgery.
Operative procedure
The operation procedures were based on the Pittsburgh technique (12) with slightly modification. All patients underwent thoracoscopic esophageal mobilization and lymphadenectomy, laparoscopic gastric mobilization, lymphadenectomy and gastric tube formation, and cervical anastomosis. In order to avoid the selection bias, both two different techniques were performed by Prof. Hongwei Zhang, the director of our department.
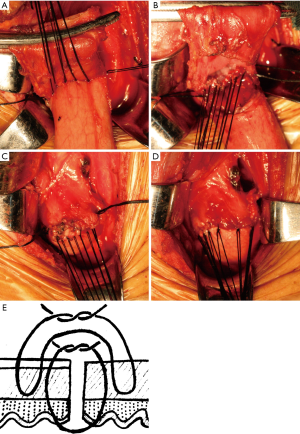
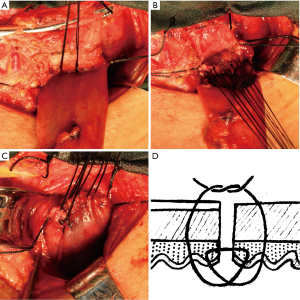
During gastric tube formation, gastroepiploic vessels and three branches of the right gastric artery were preserved to ensure sufficient blood supply of gastric tube. In detail, the lymph nodes along the right gastric artery were removed. The vessels between the third branch and fourth branch were ligated and cut. The gastric was cut from the point between the third branch and fourth branch of right gastric artery to the gastric fundus using cutting closure device. The proximal end of the gastric tube was resected as much as possible to ensure the good vascularity. The gastric tube was anticlockwise rotated for about 30° to ensure the stapling line at the anastomotic stoma was located at the anterior wall of gastric tube. In the Albert-Lembert suture group (Figure 1), interrupted posterior seromuscular sutures were performed using 3-0 silk to approximate the gastric tube and esophagus with 3 mm interval (Figure 1A). Then, interrupted stitches with full-thickness of the esophagus and gastric tube were performed (Figure 1B). The anterior wall of the anastomosis was performed in the same pattern as that of the posterior wall (Figure 1C). Finally, the anterior anastomosis was over sewn with interrupted 3-0 silk (Figure 1D). In the hybrid-layered suture group (Figure 2), interrupted posterior seromuscular sutures were performed using 3-0 silk to approximate the posterior wall of gastric tube and esophagus with three stitches (Figure 2A). Then, the needle goes from the fiber membrane through all layers into the lumen of the posterior wall of esophagus, across the incision, goes into the mucosal surface of the gastric tube and out from the submucosa, across the incision, goes into the submucosa of the esophagus and out from the mucosa, across the incision, goes from the mucosa through all layers and out from the serosa of gastric tube (Figure 2B,D). The anterior wall of the anastomosis was performed in the same pattern as that of the posterior wall (Figure 2C,D).
Postoperative management
Postoperatively, enteral nutrition was administrated on the postoperative day (POD) 1 through jejunal feeding tube. The amount of enteral nutrition was gradually increased if there was no nausea, vomiting, abdominal distension, abdominal pain and diarrhea. The drainage in the neck was removed on POD 3 or POD 4. Upper gastrointestinal water-soluble contrast examination was performed on POD 5 to exam the integrity of the anastomosis. The oral feeding was initiated after the demonstration of no anastomotic leak from water to liquid to semi-liquid diet. If anastomotic leakage was identified, the cervical wound was opened to ensure adequate drainage of the collection or abscess. If the leakage demonstrated by contrast study without any clinical symptoms, the patients were treated conservatively without opening of the cervical wound.
Follow up
Majority of the patients were discharged between POD 7 and POD 10. All the patients were followed 1 week after discharge, and were seen every 3 months. Routine blood test, liver function test, upper gastrointestinal water-soluble contrast examination, endoscopy, and CT were performed in all patients.
Postoperative complications
Anastomotic leakage was defined according to the extravasation of water-soluble contrast examination or the extravasation of collections from cervical drainage or cervical wound. Anastomotic stricture was defined by dysphagia complaint or endoscopic proof of a stenosis which required dilatation or placement of stent to relieve postoperative dysphagia. Anastomotic recurrence was ruled out by endoscopic biopsy. If the patient complained about dysphagia between two follow up time points, endoscopic examination was routinely performed. If the patient was able to swallow only semisolid foods or had worse symptoms, dilatation of the stricture was performed by endoscopy. The degree of anastomotic leakage and anastomotic stricture were identified according to the Clavien-Dindo classification (13). Grade I, any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic, and radiological interventions. Allowed therapeutic regimens are: drugs as antiemetics, antipyretics, analgetics, diuretics, electrolytes, and physiotherapy. This grade also includes wound infections opened at the bedside. Grade II, requiring pharmacological treatment with drugs other than such allowed for Grade I complications. Blood transfusions and total parenteral nutrition are also included. Grade IIIa, intervention not under general anesthesia. Grade IIIb, intervention under general anesthesia. Grade IVa, single organ dysfunction (including dialysis). Grade IVb, multiorgan dysfunction. Grade V, death of a patient.
Statistical analysis
Data were processed using SPSS 16.0 for Windows. Numerical variables were expressed as the mean (range) unless otherwise stated. Differences between the two groups were tested using a two-tailed Student’s t-test. Discrete variables were analyzed using chi-square test or fisher’s exact test. The P value <0.05 was considered statistically significant.
Results
Clinical characteristic
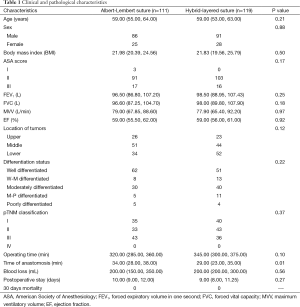
We retrospectively analyzed 230 ESCC patients underwent esophagectomy with CEGA from November 2010 to September 2012. Among them, 111 patients underwent Albert-Lembert suture and 119 patients underwent hybrid-layered suture. The perioperative clinical characteristics of the two groups were compared in Table 1. There were no significant differences between two groups in age, sex, body mass index (BMI), American Society of Anesthesiology (ASA) score, smoking history, forced expiratory volume in one second (FEV1), forced vital capacity (FVC), maximum ventilatory volume (MVV), ejection fraction (EF) value, location of tumors, differentiation status, pTNM classification, operating time, blood loss and postoperative stay (all P>0.05). The time of anastomosis in the hybrid-layered suture group [29.00 (23.00, 35.00) min] was significantly shorter than that in Albert-Lembert suture group [34.00 (28.00, 38.00) min, P=0.02].
Full table
Anastomotic leakage
Anastomotic leakage occurred in 14 patients in the Albert-Lembert suture group (12.61%), four cases with Grade I leakage, five cases with Grade II leakage, four cases with Grade IIIa leakage and one case with Grade IIIb leakage (Table 2). Compared with Albert-Lembert suture group, anastomotic leakage occurred in four patients in the hybrid-layered suture group (3.36%, P=0.01), three cases with Grade I leakage and one case with Grade IIIa leakage. There were no significant differences between the two groups regarding the distribution of anastomotic leakage classes (P=0.33). Of all 18 patients with anastomotic leakage, 17 patients were treated conservatively with gastrointestinal decompression, fasting, antibiotics and adequate drainage, 1 patient underwent jejunostomy.

Full table
Anastomotic stricture
Benign anastomotic stricture confirmed by endoscopy developed in 15 patients (13.51%) in Albert-Lembert suture group and in 6 patients (5.04%) in the hybrid-layered suture group. The onset of stricture showed no significant differences between the two groups (Table 2). All the patients with anastomotic strictures were treated with dilatation. The frequency of dilatations needed in the Albert-Lembert suture group were 1.47±0.24 times, compared with 1.67±0.33 times in the hybrid-layered suture group (P=0.64).
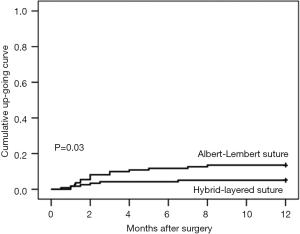
We found that anastomotic stricture developed in 5 of 18 patients who had anastomotic leak (27.77%), and in 16 of 212 patients without anastomotic leak (7.55%, P=0.02). There was no significant difference between the two groups in this relation between anastomotic leak and stricture, 4 of the 14 patients with anastomotic leak in the Albert-Lembert suture group developed stricture (28.57%) compared to 1 of 4 patients with anastomotic leak in the hybrid-layered suture group (25.00%, P=1.00). Using the Kaplan-Meier method, the rates of stricture in both groups are plotted in Figure 3. The one year cumulative stricture rate was 13.51% in the Albert-Lembert suture group and 5.04% in the hybrid-layered suture group (P=0.03).
Discussion
Considering the frequent and still important problem of CEGA leakage and stricture after esophagectomy, a successful anastomosis is critical to the favorable outcome of esophagogastrectomy. Although traditional hand sewn CEGA technique has been widely used for treatment of ESCC, the optimal CEGA technique remains subject of debate.
In several studies, the hand sewn ETE CEGA was associated with 4-25% anastomotic leaks (11). In our present study, anastomotic leak rate was 7.83%, which was relatively low compared to majority of the previous reports. The low incidence of anastomotic leak in our present study could be attributed to series of factors. Previous studies reported that the volume of centers and surgeons are also associated with anastomotic leakage (14,15). More than 120 cases of esophagectomy were performed in our department per year and every single surgeon could perform more than 50 cases of esophagectomy per year. The high center volume (16) and surgeon specialty of our department may resulted in the low anastomotic leak rate. The other main reason could be the preparation of gastric tube. A gastric tube with 3 cm wide was formed by resection along the lesser curvature using linear stapler device. The reported formation of gastric tube mostly relies right and left gastroepiploic artery as the only arterial blood supply (11). As approximately 15% of patients with a Kosk as type II anatomy lack connections between the two arteries (17,18), therefore preservation of these two arteries may resulted in ischemia of gastric tip, which may lead to anastomotic leak. Thus, three branches of the right gastric artery were preserved during gastric tube formation in addition to the preservation of right and left gastroepiploic artery. This procedure could partially improve blood supply of gastric tube and may prevent anastomotic leak.
In our present study, the leak rate in the hybrid-layered suture group was 3.36%, which was significantly lower than that in the Albert-Lembert suture group (12.61%). Although hypertension, diabetes mellitus, chronic obstructive pulmonary disease, heart disease and lower FEV1% are considered as risk factors for developing anastomotic leakage (19-21), these parameters were comparable between the two groups. Thus, the different leak rate mainly attributed to the different anastomotic techniques.
Dysphagia after esophagectomy could be resulted from several reasons including anatomic and functional etiologies. Dysphagia alone does not prove the presence of anastomotic stricture, and not all patients with anastomotic stricture complain about dysphagia. Therefore, endoscopic examination was performed immediately if patients complain about dysphagia, or routinely performed on follow up time points. In the previous studies, hand sewn ETE CEGA was associated with 9-45% anastomotic stricture formation (11). In our present study, the overall anastomotic stricture rate was 9.13%. The variation of anastomotic stricture rate reported in the literatures may be influenced by series factors including the diagnostic criteria (22) of anastomotic stricture and follow up duration. Mathew et al. reported that excessive tension on the gastrointestinal anastomosis may promote stricture formation (23). During anastomosis in both of our groups, the gastric tube was fixed to the esophageal hiatus and thoracic inlet through three interrupted stitches, which could prevent excessive tension on the anastomosis resulted from descending of the gastric tube, and finally may reduce stricture formation.
In the present study, the anastomotic stricture developed in 6 of 119 patients (5.04%) in hybrid-layered suture group, which was significantly lower than that of Albert-Lembert suture group (13.51%). Anastomotic strictures are mainly associated with the following factors including anastomotic technique, surgeon specialty and anastomotic leakage (24). Poor involution of mucosa and muscularis of gastric tube and esophagus may result in scar formation, which could promote anastomotic stricture formation (25). Thus, we considered that low anastomotic stricture rate in hybrid-layered suture group may partially attribute to the well involution of mucosa and muscularis of the anastomotic stoma. Double-layer suture in our Albert-Lembert suture group is another potential risk factor of anastomotic stricture formation, as double-layer suture could decrease the inner diameter of anastomosis (26).
Anastomotic leakage following CEGA is a known risk factor for development of anastomotic stricture, which has been reported to occur in approximately 50% of anastomotic leaks (27). In our present study, anastomotic stricture developed in 5 of 18 patients who had anastomotic leak (27.77%), and there was no significant difference in this relation between the two groups. We also found that patients with anastomotic leakage were prone to develop anastomotic stricture subsequently.
We found that hybrid-layered suture was quicker to perform than Albert-Lembert suture. The main reason for this shorter time was probably because hybrid-layered suture was performed using one layer suture rather than double layer suture in Albert-Lembert suture group. However, no significant difference in total operation time was observed between the two groups. We found no significant difference in the time to onset of stricture and average dilatations between the two groups. The frequency of dilatations needed to treat anastomotic stricture in our present study was lesser than that reported by Nederlof et al. (11). After the last dilatation, considering economic factors, many patients did not come back for further dilatation if anastomotic stricture progressed. This could partially affect the frequency of dilatations recorded.
The hybrid-layered suture method in our study was similar to Gambee suture method (28). In 1951 Gambee designed a suture method that apposed both the serosa and the mucosa, forming a single layer anastomosis (29). The Gambee suture was performed by passing the needle from the serosa through all layers into the lumen. Then, the needle was directed from the lumen through the submucosa, across the incision, through the submucosa and mucosa and into the lumen. Then, the suture was reintroduced through the entire thickness of the wall to emerge on serosal surface of the bowel (30). Gambee suture was found less edema in the anastomotic line and better mucosal coverage, and it histologically was closer to the ideal healing (31). Thus, Gambee suture seemed to be an ideal technique for anastomosis (32). However, we found that Gambee suture was slightly less easy to perform. Thus, we introduced a new suture method in our clinical practice. Unfortunately, the new suture method was not compared with Gambee suture method in our study.
There are limitations to our study. First, it was a retrospective study. To clarify the influence of suture methods on anastomotic leakage and stricture, a well-designed randomized clinical trial should be carried out. Second, the sample size of the current study was fairly small, and further study with larger sample size should be carried out to confirm our findings. Third, further studies comparing the hybrid-layered suture to Gambee suture should be carried out.
Conclusions
In conclusion, hand sewn ETE CEGA with hybrid-layered suture is associated with lower anastomotic leakage and stricture rate, compared to hand sewn ETE CEGA with Albert-Lembert suture.
Acknowledgements
Funding: This study was supported in part by grants from the National Natural Scientific Foundation of China (No. 31100643, 31570907, 81572306, 81502403).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ye T, Sun Y, Zhang Y, et al. Three-field or two-field resection for thoracic esophageal cancer: a meta-analysis. Ann Thorac Surg 2013;96:1933-41. [PubMed]
- Gao Y, Wang Y, Chen L, et al. Comparison of open three-field and minimally-invasive esophagectomy for esophageal cancer. Interact Cardiovasc Thorac Surg 2011;12:366-9. [PubMed]
- Huang HT, Wang F, Shen L, et al. Clinical Outcome of Middle Thoracic Esophageal Cancer with Intrathoracic or Cervical Anastomosis. Thorac Cardiovasc Surg 2015;63:328-34. [PubMed]
- Klink CD, Binnebösel M, Otto J, et al. Intrathoracic versus cervical anastomosis after resection of esophageal cancer: a matched pair analysis of 72 patients in a single center study. World J Surg Oncol 2012;10:159. [PubMed]
- Behzadi A, Nichols FC, Cassivi SD, et al. Esophagogastrectomy: the influence of stapled versus hand-sewn anastomosis on outcome. J Gastrointest Surg 2005;9:1031-40; discussion 1040-2.. [PubMed]
- Nguyen NT, Rudersdorf PD, Smith BR, et al. Management of gastrointestinal leaks after minimally invasive esophagectomy: conventional treatments vs. endoscopic stenting. J Gastrointest Surg 2011;15:1952-60. [PubMed]
- Saluja SS, Ray S, Pal S, et al. Randomized trial comparing side-to-side stapled and hand-sewn esophagogastric anastomosis in neck. J Gastrointest Surg 2012;16:1287-95. [PubMed]
- Deng B, Wang RW, Jiang YG, et al. Functional and menometric study of side-to-side stapled anastomosis and traditional hand-sewn anastomosis in cervical esophagogastrostomy. Eur J Cardiothorac Surg 2009;35:8-12. [PubMed]
- Lerut T, Coosemans W, Decker G, et al. Anastomotic complications after esophagectomy. Dig Surg 2002;19:92-8. [PubMed]
- Hsu HH, Chen JS, Huang PM, et al. Comparison of manual and mechanical cervical esophagogastric anastomosis after esophageal resection for squamous cell carcinoma: a prospective randomized controlled trial. Eur J Cardiothorac Surg 2004;25:1097-101. [PubMed]
- Nederlof N, Tilanus HW, Tran TC, et al. End-to-end versus end-to-side esophagogastrostomy after esophageal cancer resection: a prospective randomized study. Ann Surg 2011;254:226-33. [PubMed]
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238:486-94; discussion 494-5. [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [PubMed]
- Viklund P, Lindblad M, Lu M, et al. Risk factors for complications after esophageal cancer resection: a prospective population-based study in Sweden. Ann Surg 2006;243:204-11. [PubMed]
- Dimick JB, Pronovost PJ, Cowan JA, et al. Surgical volume and quality of care for esophageal resection: do high-volume hospitals have fewer complications? Ann Thorac Surg 2003;75:337-41. [PubMed]
- Feng MX, Wang H, Zhang Y, et al. Minimally invasive esophagectomy for esophageal squamous cell carcinoma: a case-control study of thoracoscope versus mediastinoscope assistance. Surg Endosc 2012;26:1573-8. [PubMed]
- Ndoye JM, Dia A, Ndiaye A, et al. Arteriography of three models of gastric oesophagoplasty: the whole stomach, a wide gastric tube and a narrow gastric tube. Surg Radiol Anat 2006;28:429-37. [PubMed]
- Buunen M, Rooijens PP, Smaal HJ, et al. Vascular anatomy of the stomach related to gastric tube construction. Dis Esophagus 2008;21:272-4. [PubMed]
- Cooke DT, Lin GC, Lau CL, et al. Analysis of cervical esophagogastric anastomotic leaks after transhiatal esophagectomy: risk factors, presentation, and detection. Ann Thorac Surg 2009;88:177-84; discussion 184-5. [PubMed]
- Tabatabai A, Hashemi M, Mohajeri G, et al. Incidence and risk factors predisposing anastomotic leak after transhiatal esophagectomy. Ann Thorac Med 2009;4:197-200. [PubMed]
- Mine S, Udagawa H, Tsutsumi K, et al. Colon interposition after esophagectomy with extended lymphadenectomy for esophageal cancer. Ann Thorac Surg 2009;88:1647-53. [PubMed]
- Williams VA, Watson TJ, Zhovtis S, et al. Endoscopic and symptomatic assessment of anastomotic strictures following esophagectomy and cervical esophagogastrostomy. Surg Endosc 2008;22:1470-6. [PubMed]
- Mathew A, Veliuona MA, DePalma FJ, et al. Gastrojejunal stricture after gastric bypass and efficacy of endoscopic intervention. Dig Dis Sci 2009;54:1971-8. [PubMed]
- Davis SJ, Zhao L, Chang AC, et al. Refractory cervical esophagogastric anastomotic strictures: management and outcomes. J Thorac Cardiovasc Surg 2011;141:444-8. [PubMed]
- Takata MC, Ciovica R, Cello JP, et al. Predictors, treatment, and outcomes of gastrojejunostomy stricture after gastric bypass for morbid obesity. Obes Surg 2007;17:878-84. [PubMed]
- Nguyen NT, Stevens CM, Wolfe BM. Incidence and outcome of anastomotic stricture after laparoscopic gastric bypass. J Gastrointest Surg 2003;7:997-1003; discussion 1003. [PubMed]
- Orringer MB, Marshall B, Iannettoni MD. Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 2000;119:277-88. [PubMed]
- Gambee LP, Garnjobst W, Hardwick CE. Ten years’ experience with a single layer anastomosis in colon surgery. Am J Surg 1956;92:222-7. [PubMed]
- Gambee LP. A single-layer open intestinal anastomosis applicable to the small as well as the large intestine. West J Surg Obstet Gynecol 1951;59:1-5. [PubMed]
- Auletta L, Lamagna F, Uccello V, et al. In vitro comparison of three suture techniques for anastomosis of the equine small intestine. Equine Vet J Suppl 2011.46-50. [PubMed]
- Krasniqi A, Gashi-Luci L, Krasniqi S, et al. A comparison of three single layer anastomotic techniques in the colon of the rat. Int J Surg 2009;7:31-5. [PubMed]
- Hirata K, Konishi T, Ueda Y, et al. Healing in the intestinal anastomosis--comparison of the Albert-Lembert and Gambee methods. J UOEH 2000;22:1-6. [PubMed]


