Caution! Overestimation of treatment effects of corticosteroid therapy for community-acquired pneumonia in a meta-analysis of randomized controlled trials
Meta-analyses aim to increase the power and precision of the estimated intervention effects. Meta-analyses of high-quality randomized clinical trials (RCTs) are generally considered the highest level of evidence for intervention effects (1). However, when the relevant evidence is limited, meta-analyses are often underpowerd to establish realistic intervention effect estimates. Of particular note is that when meta-analyses include a limited number of patients and a small number of events, overestimation of intervention effect estimates may occur and could cause spurious results (2). Random error often is the more frequent cause for the overestimation. To overcome the issue, trial sequential analysis (TSA) is introduced to project the required information size (RIS) for meta-analyses (3), which can explore the independent effect of random error on intervention effect estimates in meta-analyses and protect meta-analyses against overestimation due to random error.
Siemieniuk and colleagues investigated the effect of corticosteroid therapy on mortality and morbidity in adults with community-acquired pneumonia (CAP) and concluded that corticosteroid therapy may reduce all-cause mortality by approximately 3%, need for mechanical ventilation by approximately 5%, and duration of hospitalization by approximately 1 day (4). The authors should be commend for their excellent and important work. However, I believe that the conclusion requires further comments. In this study, there are few events and limited trials for many outcomes, as acknowledged by the authors. Thus, overestimation of treatment effects of corticosteroid therapy for CAP is inevitable, and potentially spurious evidence of effects may exist. Here, illustrating with example of one of many outcomes (i.e., mortality), I apply TSA to determine whether the evidence in this meta-analysis is reliable and conclusive. I calculated the RIS to yield “moderate” meta-analytic evidence based on an α=0.05 (two sided), β=0.20 (power 80%), an anticipated relative risk reduction of 20%, and an event proportion of 7.9% in the control arm. TSA on mortality showed the RIS (9,251 patients) is not reached, with the absence of reliable and conclusive evidence, as shown in Figure 1. Similarly, using TSA on other outcomes, the corresponding RIS also is not reached (not shown here).
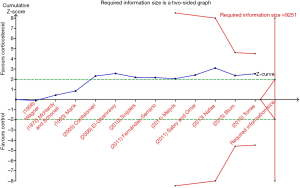
In summary, the treatment effects of corticosteroid therapy for CAP may be inflated, which limits the strength of the inferences that can be drawn. The current evidence on corticosteroid therapy for CAP is insufficient and inconclusive, and further trials are desirable to obtain firm and reliable evidence.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, 2011. Available online: http://www.cochrane.org/handbook
- Thorlund K, Imberger G, Walsh M, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta-analysis--a simulation study. PLoS One 2011;6:e25491. [PubMed]
- Thorlund K, Engstrøm J, Wetterslev J, et al. User manual for trial sequential analysis (TSA). Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark. 2011:1-115. Available online: www.ctu.dk/tsa
- Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid Therapy for Patients Hospitalized With Community-Acquired Pneumonia: A Systematic Review and Meta-analysis. Ann Intern Med 2015;163:519-28. [PubMed]


