Ultrasound techniques in the evaluation of the mediastinum, part 2: mediastinal lymph node anatomy and diagnostic reach of ultrasound techniques, clinical work up of neoplastic and inflammatory mediastinal lymphadenopathy using ultrasound techniques and how to learn mediastinal endosonography
Introduction
For a thorough mediastinal nodal evaluation including tissue sampling, a variety of techniques are available: endoscopic techniques (e.g., bronchoscopy), radiological methods (e.g., computed tomography, fluoroscopy, and magnetic resonance imaging), nuclear medicine techniques (e.g., positron emission tomography) and surgical procedures (e.g., mediastinoscopy and video-assisted thoracoscopy). Additionally ultrasound-derived techniques have been introduced that have changed the workflow in the evaluation of mediastinal diseases. Endobronchial ultrasound combined with transbronchial needle aspiration (EBUS-TBNA) and endoscopic ultrasound fine needle aspiration (EUS-FNA) have replaced surgical staging as the initial test of choice for mediastinal tissue evaluation (1-15). Regardless of its numerous advantages, ultrasound-derived techniques are still not utilized to their full potential in respiratory medicine.
The aim of this review in two integrative parts is to discuss the current role and future perspectives of ultrasound techniques for staging of lung cancer and for the evaluation of mediastinal lymphadenopathy. Part 1 deals with an introduction into ultrasound techniques, and part 2 does with the mediastinal lymph node (MLN) anatomy and diagnostic reach of ultrasound techniques, the clinical work up of neoplastic and inflammatory mediastinal lymphadenopathy using ultrasound techniques and how to learn mediastinal endosonography.
MLN anatomy and diagnostic reach of ultrasound techniques
To ensure efficient performance of all mediastinal ultrasound techniques, it is important to have a profound knowledge of mediastinal anatomy and insight how ultrasound images relate to the different nodal stations. According to the International Association for the Study of Lung Cancer (IASLC) classification MLN are divided into different lymph node regions (16). A more anatomically detailed description is given in the following paragraph. The supra-aortic region is defined as the compartment directly above the aortic arch, excluding the area posterior to the trachea, the right paratracheal region as the compartment anterior and lateral to the trachea below the brachiocephalic trunk and above the right bronchus, the aortopulmonary window as the compartment below the aortic arch and above pulmonary trunk, left pulmonary artery, and left main bronchus, the prevascular region as the compartment anterior to the ascending aorta, vena cava superior, and pulmonary trunk and behind the upper sternum, and the pericardial region as the compartment anterior and lateral to the heart. In the following paragraphs and Table 1 mediastinal lymph node stations and their evaluability by ultrasound techniques are summarized.
Full table
MLN evaluation by (transesophageal) endoscopic ultrasound (EUS)
EUS-guided biopsy allows excellent LN evaluation mainly of the lower mediastinum including the subcarinal region 7, paraesophageal region 8 and pulmonary ligament region 9. EUS also allows access to the left paratracheal region (4L) and partially to the left hilar region (10L). EUS-FNA of region 5 is safe and effective if lymph nodes are considerably enlarged whereas in small lymph nodes FNA might be more difficult or impossible due to interposition of the pulmonary artery/aorta. The para-aortal lymph nodes (station 6) are even more difficult to assess; biopsy (from above the aortic arch) is often difficult avoiding the large mediastinal vessels (17), and the transaortic approach using a 25G-needle may be used only in selected cases (18). The right sided paratracheal and hilar located LN (2R, 4R, 10R) can only be evaluated when grossly enlarged. This can be explained by the anatomy of the esophagus which is located posterior and left-sided to the air-guiding trachea. Therefore, the trachea prevents visualization of the right sided mediastinal regions (19). The examination technique has been recently summarized in textbooks (20-22).
MLN evaluation by endobronchial ultrasound (EBUS)
EBUS-guided biopsy allows excellent LN evaluation of the right (2R, 4R) and left-sided (2L, 4L) paratracheal and the subcarinal regions (7). In addition EBUS provides also easy bilateral access to the hilar region 10 and to the interlobar region 11. Access to intrapulmonary lymph node regions 12-14 is possible only using radial mini-probes (EBUS-R) (23).
In conclusion EBUS and EUS allow complementary evaluation of almost all MLN localizations and combining both methods virtually all mediastinal nodes can be sampled.
MLN evaluation by transcutaneous mediastinal ultrasound (TMUS)
TMUS allows the standardized examination of the supra-aortic region, prevascular region, right sided upper and lower paratracheal regions (regions 2R, 4R), aortopulmonary window (region 5) and subcarinal region (region 7) under most circumstances (24-30). In addition the precardial region can be easily evaluated.
Clinical work up of mediastinal lymphadenopathy using ultrasound techniques
Enlargement of MLNs is a frequent finding in inflammatory and neoplastic diseases. Conventional chest radiography and thoracic computed tomography are first line diagnostic methods to evaluate suspected mediastinal lymphadenopathy (2,31). In addition, ultrasound methods have gained importance mainly due to their ability to guide biopsy and interventions but also to their detailed spatial resolution. Ultrasound methods allow not only size-related criteria as shown for computed tomography and magnetic resonance imaging but also evaluation of the lymph node architecture (32,33), lymph node vascularity and perfusion (34-37), resistance index (38), lymph node elasticity (39-41) and changes of perfusion under antiangiogenetic treatment (34).
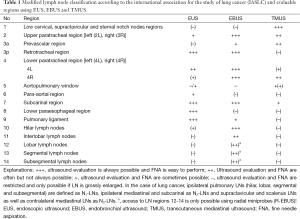
Several studies have tried to define typical ultrasound criteria for malignant MLNs. One North American and one European EUSstudy found MLNs in 86% and 62% of patients with benign diseases and healthy individuals (42,43). Almost all of these normal lymph nodes have a short diameter below 10 mm and a triangular, crescent or oval shape. Other features like homogeneity, central echogenic structure, and contour differed between individuals and nodes. Contrary to what is often claimed, in both studies number and size of MLNs did not differ between smokers and non-smokers (42,43). Catalano et al. (44) in 1994 in a cohort of 100 patients with esophageal cancer defined endosonographic features predictive of lymph node metastasis: hypo- echoic structure, distinct margin, roundness, and a diameter greater than 10 mm. Additionally, as the number of “malignant” echo features rises, the probability of malignancy increased. Malignancy could be predicted with 100% accuracy when all four features were present (44). These endosonographic criteria have been confirmed in further studies using EUS and EBUS. Additional predictive criteria for malignancy of lymph nodes have been added: absence of echogenic hilar structure and of central nodal vessel, echogenic coagulation necrosis, heterogeneous echo pattern (33,45-49). However, a definitive classification as either malignant or benign by endosonographic criteria is possible only in approximately 25% of MLNs (50). Classification of lymph nodes by EUS criteria alone is less reliable in mediastinal than in lymph nodes of other anatomical locations. Therefore, especially in MLNs EUS-guided fine needle aspiration (FNA) has a significantly higher accuracy than echo features alone (51) (Figures 1,2). However, the probability of malignancy is very low, if none of the malignant lymph node criteria is observed (33,52,53).
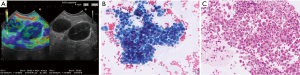
From a practical point of view it was suggested that patients without any pathological sonographic lymph node criteria should not be biopsied whereas all other lymph nodes should be considered for biopsy (53).
A summary of possible indications for ultrasound techniques in the evaluation of mediastinal and lung diseases is summarized in Table 2 (8,23,54).

Full table
Lung cancer
Lung cancer is one of the most common malignancies and accounts for very high cancer related mortality. In the absence of distant metastases, MLN staging is the most important factor that affects the management and prognosis of patients with lung cancer. Knowledge of locoregional tumor stage is important for planning the best choice of treatment including surgical resection, radiation and chemotherapy (2,31). Mediastinoscopy and thoracoscopy are invasive techniques and, therefore, should be avoided if not necessary.
Lymph node staging
It is obvious that there is an increasing need for minimally invasive techniques including EBUS and EUS techniques with needle aspiration for MLN staging. It has to be taken into account that results of transbronchial biopsy techniques (TBNA) relying on “blind” biopsy are disappointing (55). EBUS-TBNA has significantly improved the biopsy results (56-62). Promising results have been shown in a multicentric study of 502 patients with a mean lymph node diameter of 16 mm. The reported sensitivity was 94%, specificity 100%, and the positive predictive value 100% (63). Recent meta-analyses have shown a pooled sensitivity of EBUS-TBNA in the range of 88% to 93% (2,64-67). EUS-FNA has a comparable diagnostic yield. Two recent meta-analyses report on a pooled sensitivity of EUS-FNA in nodal staging of NSCLC of 83% and 89%, respectively (2,68).
EUS-FNA and EBUS-TBNA have a complementary diagnostic reach. In combination with both EUS and EBUS almost all important MLNs can be biopsied (59,62,69). Several studies have shown that a combined EUS- and EBUS-approach (“complete endosonographic mediastinal staging”) improves lymph node staging versus each of the techniques alone (7-10,23). The sensitivity of both, EUS and EBUS for MLN staging is around 90% (2,23,67,70-73). Meta-analytic data show a substantial increase in sensitivity for mediastinal nodal staging in patients with proven or suspected lung cancer by combining EBUS-TBNA and EUS-FNA or transesophageal FNA using an EBUS-bronchoscope (EUS-B-FNA). Average increment in sensitivity was 21% compared with the esophageal approach alone (pooled data from seven studies) and 13% compared with EBUS-TBNA alone (pooled data from nine studies) (5). Similar data were reported in another meta-analysis including only studies comparing EBUS-TBNA and EUS-B-FNA with an increase in sensitivity for lung cancer staging of 11% by combining both techniques vs. EBUS-TBNA alone (74). The accuracy of the combined approach using EUS-FNA plus EBUS-TBNA proved to be significantly higher than that of PET-CT alone (90.0% vs. 73.6%) (12). A randomized controlled study comparing two approaches to combined endosonographic mediastinal staging (EBUS first vs. EUS first) found no differences of efficacy and patient’s satisfaction in both groups. However, EBUS-TBNA turned out to be the more efficient primary procedure in endoscopic mediastinal staging of potentially operable lung cancer (13). The published studies mainly include cohorts of patients with a relatively high prevalence of mediastinal lymphadenopathy (median 58% for EUS-FNA and EBUS-TBNA; 33% for the combined approach) (2). In studies with a low prevalence of MLN metastases sensitivity of both endosonographic techniques was considerably lower than in studies with a high prevalence (2). Moreover, it has to be taken into account that enlarged lymph nodes and PET-positive findings are the inclusion criteria for most published studies. Meta-analyses uniformly show that sensitivity of EUS-FNA and EBUS-TBNA for detection of metastatic invasion in patients selected on the basis of CT or PET positive results is significantly higher than in patients with negative CT findings or without any selection of CT or PET (66,68). This underlines the importance of biopsies to identify patients who need neoadjuvant treatment strategies.
The combined use of EUS and EBUS can prevent >50% of scheduled surgical staging procedures by providing tissue proof of advanced disease in patients with suspected lung cancer and enlarged or PET positive lymph nodes (75,76).
The results for re-evaluation after neoadjuvant treatment are generally more skeptical (less than 75%). Due to the reported low negative predicative value, negative lymph node findings should be surgically verified (8).
However, both EBUS and EUS have limitations in excluding malignant lymph node involvement. False negative EUS and EBUS findings occur either to sampling errors (lymph node found and biopsied but metastasis missed) or a detection error (lymph node not found).
EUS and EBUS vs. mediastinoscopy
Mediastinoscopy, a surgical staging procedure, has been regarded as the gold standard for a long period of time with a sensitivity of 78% for mediastinal nodal staging (72). The additional use of EUS to mediastinoscopy improved locoregional staging (cT4N2-3). The improved results were explained by the complementary diagnostic reach of various nodal stations and the ability of EUS to assess mediastinal tumor invasion (73). The use of mediastinoscopy after a negative endosonography improved the sensitivity of mediastinal nodal staging from 85% to 94% (8). The question which patients staged negative by endosonography should subsequently undergo surgical staging of the mediastinum is a matter of current discussions. It is recommended that in patients with suspicious lymph nodes on either CT or PET, negative endosonography findings should be surgically verified. In contrast there is evidence that patients with centrally located tumors or suspected hilar abnormalities do not benefit from additional surgical staging (23). However, in a very recent study in patients with suspected single level N1 disease, the sensitivity of EBUS for N2 disease was disappointingly low (38%) (77) suggesting a role for mediastinoscopy. However, in this cohort often only EBUS and not the EBUS-EUS combination was used.
It could be shown that in 10-25% of patients with negative CT and in 5-10% of patients with negative PET subsequent endosonographic examinations verify lymph node metastases (78-81).
In conclusion, a complete endosonography evaluation of the mediastinum is at least as good as mediastinoscopy but is associated with fewer complications and futile thoracotomies (8). Therefore, endosonography (and not mediastinoscopy) qualifies as the initial mediastinal tissue staging test (31). Negative endosonography findings however should be verified by surgical staging (5,82).
Proof of diagnosis
In about one third of patients with suspected lung cancer, conventional bronchoscopy fails to prove the diagnosis. In patients with suspected lung carcinoma adjacent to the trachea or bronchi without mucosal (endobronchial) abnormalities, EBUS is superior to CT for guidance of biopsy. The reasons include a better diagnostic yield and a much lower rate of complications including pneumothorax and bleeding in the case of perivascular tumor growth (83,84). In addition, EUS can be used to biopsy centrally located intrapulmonary periesophageal tumors if conventional methods fail (71). In a group of 123 patients with an undiagnosed but suspected malignant lung lesion (paratracheal, parabronchial, paraesophageal) or with a peripheral lung nodule and PET-positive MLNs who had undergone at least one diagnostic flexible bronchoscopy or CT-guided transthoracic needle aspiration attempt, EBUS-TBNA and/or EUS-FNA had a high diagnostic efficacy. The endosonographic approach to diagnosis of lung cancer avoided expensive surgical procedures in 106 cases and led to significant cost savings (85).
T-staging
EUS is helpful in selected cases to evaluate T4 (stage IIIB) in the case of possible aortic invasion (70). This specifically applies to invasion in vascular structures. However, more data are needed to make a more definite assessment on this topic.
M-staging
Adrenal gland
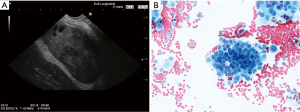
In addition, conventional EUS instruments allows the evaluation and biopsy of the left adrenal gland (86-88), which is often involved in metastatic lung carcinoma (89-93). FDG-PET uptake is helpful for detection of adrenal metastases. Focal lesions as the most important imaging sign but also increased size and loss of the typical “seagull shape” are predictors for malignant involvement (89). In addition, the left adrenal gland can be reached and sampled by EUS-FNA demonstrating a high yield (91,94,95). The right adrenal gland can be assessed using a transduodenal or transgastric approach, which is technically more demanding and sometimes dangerous if a decubitus or right sided position for visualization is required. EUS is inferior to transcutaneous ultrasound (TUS) in the evaluation of the right adrenal gland (96,97). TUS-guided biopsy is recommended (88,98). However, in cases transduodenal biopsy of suspected right sided adrenal metastases have shown to be feasible (99,100). A preoperative bilateral EUS examination and EUS-FNA of the adrenal glands in patients with potentially resectable lung cancer has a high diagnostic accuracy in detecting adrenal metastases (89,101) (Figure 3). Recently a few reports in abstract form have reported the use of the EBUS scope for the assessment of the left adrenal gland. However, more data are needed for a more definitive assessment.
Liver
In rare cases liver metastases are detectable only by EUS with CT-negative findings. In such cases the EUS-guided biopsy of the liver is helpful to proof the metastatic spread (98,102,103).
Other infradiaphragmal manifestations
In even more rare cases pancreatic metastases (or infiltration of celiac or peripancreatic lymph nodes) are detectable only by EUS with CT-negative findings. In such cases the EUS-guided biopsy of the pancreas is helpful to prove the metastatic spread (98,104-107).
Mediastinal staging of extrathoracic malignancies
As has been shown for pancreatobiliary cancer, in up to 10% of extrathoracic malignancies metastasize to MLNs (108). Both EUS and EBUS have been successfully used for the assessment of tumor spread to MLNs (M1 disease) in patient cohorts with various extra-thoracic malignant diseases (109-116). In particular, the usefulness of EUS-guided sampling of MLNs has been reported in the staging of patients with gastric cancer, pancreatic cancer (108,117) breast cancer (118), upper GI cancer (119,120); head and neck cancer (121), colorectal cancer (122), and lymphoma (57,123-129). A recent meta-analysis (five studies, n=533 patients) showed a high value for EBUS-TBNA for the diagnosis of mediastinal and hilar lymph node metastases from extrathoracic malignancy. Pooled sensitivity was estimated 85% with a specificity of 99% (130). Procurement of specimens which are eligible for immunohistochemistry is important for reliable differentiation between mediastinal nodal metastases of extrathoracic cancer vs. non-small cell lung cancer.
How to learn pneumological endosonography
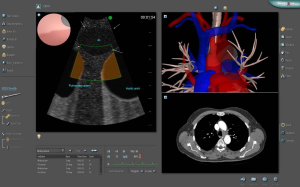
A systematic training in mediastinal endosonography should ideally be based on (I) theoretical knowledge, (II) performance on simulators and (III) supervised performance on patients. Each step should be completed by passing a validated exam before proceeding to the next step. However, there are no commercially available virtual reality simulators for mediastinal EUS-FNA, but it is possible to practice EBUS-TBNA on both the GI Bronch Mentor™ (Simbionix) (Figure 4) and the AccuTouch Flexible Bronchoscopy Simulator™ (GE Healthcare). A standardized test including pass/fail-standards has been developed for the GI Bronch Mentor (131,132).
Firstly the trainee should learn to recognize anatomic landmarks and mediastinal vessels (133) by observing the procedure (134,135). The next step is to learn to insert the endoscope and to “produce” the pictures, which is much more difficult than watching an experienced examiner doing the procedure. The final task relates to correct positioning of the transducer, proper use of the needle sheath and handling of the needle when taking fine needle aspirates (123-126,136-140).
After passing a simulation-based test the trainee should perform the initial endosonography procedures in patients under supervision. The learning curve should be monitored by specific tools for assessment, since the number of procedures to obtain competence varies from trainee to trainee (127-129,131,141,142). For EBUS-TBNA, in a multicenter cohort of fellows in pulmonary medicine, the majority of trainees achieved first independent successful performance of EBUS-TBNA following a training protocol that included theoretical education and simulation sessions at an average of only 13 procedures (143). Another study with nine interventional pneumologists failed to observe such a steep learning curve and observed ongoing improvements for lymph node identification by EBUS and EBUS-TBNA skills even after 200 clinical cases (144).
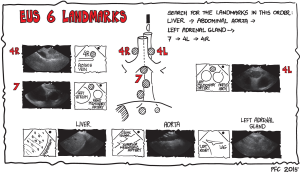
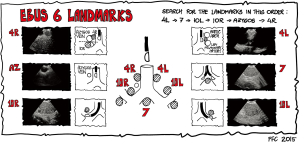
The classical approach is to start by learning the six basic landmarks for EBUS and EUS and to practice finding them in the order mentioned (Figures 4-6) (145).
The six EUS landmarks (Figure 4)
- Liver (landmark I): introduce the endoscope into the esophagus and slide down below the diaphragm. Turn the endoscope counterclockwise to find the left liver lobe.
- Aorta (landmark II): turn the endoscope clockwise and find the aorta with the celiac trunk and the superior mesenteric artery.
- The left adrenal gland (landmark III): turn the endoscope further clockwise, move the transducer a little upwards to find the left adrenal gland (it resembles a small bird, seagull) close to the upper pole of the left kidney.
- Station 7 (landmark IV): retract the endoscope to the mediastinum and find station 7 below the carina close to the left atrium and the right pulmonary artery.
- Station 4L (landmark V): retract the endoscope a few centimeters, turn counterclockwise and find station 4L between the aortic arch and the left pulmonary artery (the vessels resemble the ears of Mickey Mouse).
- Station 4R (landmark VI): turn clockwise until you pass the trachea and find the azygos vein. Retract the endoscope slowly until the vein disappears into the superior cava vein and search for station 4R. If it is of normal size, it will, often hide behind the trachea.
The six EBUS landmarks (Figure 5)
- Station 4L (landmark I): turn the endoscope counter clockwise and find station 4L between the arch of the aorta and the left pulmonary artery.
- Station 7 (landmark II) is found below the carina with the endoscope in the right or the left main bronchus facing medially.
- Station 10L (landmark III) is found looking upwards with the transducer in the left upper lobe bronchus.
- Station 10R (landmark IV) is found looking upwards with the transducer in the right upper lobe bronchus.
- The azygos vein (landmark V): retract the endoscope and find the azygos vein paratracheal to the right.
- Station 4R (landmark VI) is found above the azygos vein. The inferior border of the azygos vein marks the border between station 4R and 10R.
Handling of the endoscope: a few tips and tricks
- Note carefully on the ultrasonic picture, if the endoscope is coming from the right or the left side. “The dot” shows where the proximal part of the endoscope on the ultrasonic picture is located. Avoid confusion with a mirror image.
- With the EUS endoscope, a rotation to the right (clockwise) moves the transducer to the right side of the patient, when the transducer is directed forward, i.e., above the diaphragm. The same rotation will move the transducer to the left side of the patient when the transducer is directed backwards below the diaphragm.
- When performing EBUS, it must be remembered that the view is typically in an oblique direction of 30 degrees, so it can be difficult to get access to the trachea.
- All regions should be inspected systematically with a 360 degrees rotation for every four centimeters. Do not overlook any structures that are not necessarily located according to the two times six landmarks.
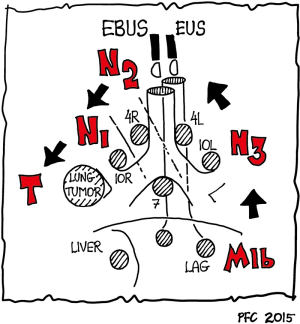
Practical advice: systematic approach to endosonographic lung cancer staging
The order of recommended examinations (EBUS, EUS) depends mainly on the side and localization of the tumor determined by the CT findings. Biopsies should be performed under the premise that distant metastases (M1) are excluded first, followed by lymph node staging in the order N3 (contralateral lymph nodes) → N2 (ipsilateral mediastinal and subcarinal lymph nodes) → N1 (ipsilateral hilar lymph nodes) (Figure 6). For patients with suspected N2 disease infiltration of only single N2 lymph nodes (N2a, stage IIIA3) has to be differentiated from infiltration of more than one N2 lymph node region, clusters of involved lymph nodes in one or more N2 stations, or large N2 lymph nodes with extracapsular invasion (N2b, Stage IIIA4) (1-3,31,72,146).
Lymphoma
Mediastinal ultrasound
In a retrospective study [40 consecutive patients with Hodgkin’s (n=29) and non-Hodgkin’s (n=11)] MTUS was clearly superior to chest radiographs and comparable to CT for monitoring patients with mediastinal lymphomas (147) (Figure 7). Thymic enlargement due to involvement by Hodgkin disease is more frequently observed than previously reported. Thymic gland involvement is sonographically visible due the hypoechoic structure. In contrast MTUS was not helpful to differentiate the normal-sized typical tongue-shaped thymus from surrounding fatty tissue after treatment due to the same echogenicity of the gland and the surrounding fat (148). Elastography and contrast enhanced techniques might overcome this problem but data are lacking (32).

It is of importance that lymphoma and other tumors in the anterior mediastinum can also be biopsied under ultrasound guidance via a suprasternal and strict parasternal approach. Using the parasternal approach non-visible lymphoma might get visible due to the shifting of the mediastinum from the decubitus to a strict left or right lateral position (22,26).
EUS-FNA and EBUS-TBNA
EUS-FNA and EBUS-FNA have a variable diagnostic yield for diagnosing and subtyping of non-Hodgkin Lymphoma of the posterior and inferior mediastinum. There are good data that EBUS/EUS is useful for the assessment of recurrent lymphoma, for the primary lymphoma diagnosis often a histology specimen—obtained by mediastinoscopy—is needed. However, cell block processing of material obtained by EUS-FNA or EBUS-TBNA may have nearly similar diagnostic yield as histology (149-152). Two large cohort studies demonstrated, that accuracy of EBUS-FBNA for diagnosis of mediastinal malignant lymphoma was 84% and 91%, with correct subtyping possible in >2/3 of cases (150,153).
Inflammatory diseases
Sarcoidosis and tuberculosis
Depending on the geographic distribution, sarcoidosis and tuberculosis are the two most important inflammatory causes of mediastinal lymphadenopathy (109,154-158).
Sarcoidosis
The typical imaging finding of sarcoidosis lymphadenopathy are symmetrically distributed clusters of MLNs around large vessels. The typically oval-shaped lymph nodes may reach a size of up to 60 mm with mixed echogenicity depending on the stage of the disease (156). Color Doppler imaging, contrast enhanced ultrasound techniques and elastography have shown that the lymph node architecture is typically not destroyed and a hilum can be displayed (159-161).
Both EUS-FNA and EBUS-TBNA are suitable for final diagnosis of sarcoidosis (Figure 8) whereas pure transbronchial biopsy fails in about one third of cases. Published data indicate that the sensitivity (80-90%) and accuracy of EUS-FNA and EBUS-TBNA are superior compared to simple mucosa biopsies without and with “blind” transbronchial puncture (155,162-165). Special techniques (cytology and cell-block analysis) might even improve the diagnostic yield of ultrasound-guided biopsies (162). A meta-analysis (14 studies including 2,097 patients) showed a diagnostic yield of 79% for diagnosis of sarcoidosis by EBUS-TBNA. Pooled sensitivity and specificity were 84% and 100%, respectively (166).
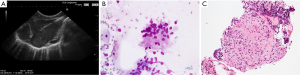
Complications may be encountered. Mediastinitis with abscess formation has been observed after transesophageal biopsy of MLNs (167). Therefore, prophylactically administered antibiotics may be considered for EUS-guided biopsies but studies on this topic are lacking. Similar complications haven not been observed in EBUS-TBNA, therefore, no prophylactically applied antibiotics are recommended.
In conclusion, for the diagnosis of sarcoidosis, endosonographic techniques are superior to the combination of endobronchial mucosa and transbronchial lymph node biopsies. Besides conventional cytological smears, cell blocks are recommended to increase the diagnostic yield.
Differential diagnoses
Under specific circumstances also depending on geographic and other epidemiological criteria tuberculosis and atypical mycobacteriosis have to be excluded in the case of unclear mediastinal lymphadenopathy. Several studies have shown acceptable diagnostic accuracy for the diagnosis of MLN tuberculosis by EUS-FNA and EBUS-TBNA. Cytopathological criteria, the search for acid-fast bacilli (stained red) using Ziehl-Neelsen-technique or Acridin-Orange-staining as well as culture techniques and PCR are helpful for final diagnosis (157,158,168-170). Concurrent systemic symptoms may be encountered (171).
Besides tuberculosis, atypical mycobacteriosis, sarcoidosis and other granulomatous diseases paraneoplastic “sarcoid like reaction” (SLR) have to be included in the differential diagnosis of granulomatous lymphadenopathy. SLR has been observed in the neighborhood of malignancies as well as sequelae of chemotherapy and radiation. Positron emission tomography (PET) may show false positive results in patients with SLR (172-174).
Mediastinal ultrasound in patients with cystic fibrosis
The respiratory tract is involved in almost all patients with cystic fibrosis and respiratory failure accounts for about 90% of morbidity and mortality in patients with cystic fibrosis. Extrapulmonary manifestations are also often encountered (175). Evaluation of TMUS in healthy subjects and patients with cystic fibrosis demonstrated that the lymph node detection rate in the paratracheal region and aortopulmonary window was significantly higher in patients with cystic fibrosis and the total lymph node volume was larger, respectively. Therefore, mediastinal ultrasound was helpful for the detection of inflammatory activity in patients with cystic fibrosis (176). Similar studies using EUS and EBUS have not been published.
Mediastinal ultrasound in patients with chronic virus hepatitis C
Mediastinal lymphadenopathy can be considered as an extrahepatic manifestation of chronic hepatitis C. TMUS was also able to detect slightly enlarged MLNs in patients with chronic virus hepatitis C. In patients with chronic hepatitis C a trend could be observed, that patients with larger perihepatic lymph nodes also reveal larger MLNs indicating a systemic pathomechanism. The mechanism of lymphadenopathy in the liver hilum (177-179) and mediastinum in patients with chronic hepatitis C and other viral and autoimmune liver diseases is yet unknown (180). Similar studies using EUS and EBUS have not been published. Therefore, normal lymph nodes were detectable more frequently in the paratracheal region and aortopulmonary window of cadavers compared to the respective mediastinal regions of healthy volunteers. A possible explanation of this finding lies in the better image resolution obtained by application of the transducer to the region of interest in cadavers. The difference in age may also have an impact.
Conclusions
Endobronchial, endoesophageal and TUS are complimentary approaches for the evaluation of the mediastinum, in particular in patients with non-small cell lung cancer and with mediastinal lymphadenopathy. All three techniques facilitate tissue acquisition from MLNs or masses for primary diagnosis or staging. Due to their high accuracy and low risk, ultrasound-guided sampling procedures should be considered to substitute for more invasive surgical techniques. Learning ultrasonographic evaluation of the mediastinum should be performed in a systematic manner based on the classical anatomical landmarks.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Detterbeck FC, Postmus PE, Tanoue LT. The stage classification of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e191S-210S.
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [PubMed]
- Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi89-98. [PubMed]
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015;47:545-59. [PubMed]
- Liberman M, Sampalis J, Duranceau A, et al. Endosonographic mediastinal lymph node staging of lung cancer. Chest 2014;146:389-97. [PubMed]
- Wallace MB, Pascual JM, Raimondo M, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA 2008;299:540-6. [PubMed]
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [PubMed]
- Herth FJ, Krasnik M, Kahn N, et al. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest 2010;138:790-4. [PubMed]
- Hwangbo B, Lee GK, Lee HS, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest 2010;138:795-802. [PubMed]
- Szlubowski A, Zielinski M, Soja J, et al. A combined approach of endobronchial and endoscopic ultrasound-guided needle aspiration in the radiologically normal mediastinum in non-small-cell lung cancer staging--a prospective trial. Eur J Cardiothorac Surg 2010;37:1175-9. [PubMed]
- Ohnishi R, Yasuda I, Kato T, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal nodal staging of lung cancer. Endoscopy 2011;43:1082-9. [PubMed]
- Kang HJ, Hwangbo B, Lee GK, et al. EBUS-centred versus EUS-centred mediastinal staging in lung cancer: a randomised controlled trial. Thorax 2014;69:261-8. [PubMed]
- Lee KJ, Suh GY, Chung MP, et al. Combined endobronchial and transesophageal approach of an ultrasound bronchoscope for mediastinal staging of lung cancer. PLoS One 2014;9:e91893. [PubMed]
- Oki M, Saka H, Ando M, et al. Endoscopic ultrasound-guided fine needle aspiration and endobronchial ultrasound-guided transbronchial needle aspiration: Are two better than one in mediastinal staging of non-small cell lung cancer? J Thorac Cardiovasc Surg 2014;148:1169-77. [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Liberman M, Duranceau A, Grunenwald E, et al. Initial experience with a new technique of endoscopic and ultrasonographic access for biopsy of para-aortic (station 6) mediastinal lymph nodes without traversing the aorta. J Thorac Cardiovasc Surg 2012;144:787-92. [PubMed]
- von Bartheld MB, Rabe KF, Annema JT. Transaortic EUS-guided FNA in the diagnosis of lung tumors and lymph nodes. Gastrointest Endosc 2009;69:345-9. [PubMed]
- Tournoy KG, Annema JT, Krasnik M, et al. Endoscopic and endobronchial ultrasonography according to the proposed lymph node map definition in the seventh edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2009;4:1576-84.
- Dietrich CF. Endosonographie. Lehrbuch und Atlas des endoskopischen Ultraschalls. Stuttgart: Thieme Verlag, 2008.
- Dietrich CF. Endoscopic ultrasound, an introductory manual and atlas. Second edition. Stuttgart: Thieme Verlag, 2011.
- Dietrich CF, Nuernberg D. Lehratlas der interventionellen Sonographie. Stuttgart: Thieme Verlag, 2011.
- Tournoy KG, Keller SM, Annema JT. Mediastinal staging of lung cancer: novel concepts. Lancet Oncol 2012;13:e221-9. [PubMed]
- Wernecke K, Peters PE, Galanski M. Mediastinal tumors: evaluation with suprasternal sonography. Radiology 1986;159:405-9. [PubMed]
- Wernecke K, Potter R, Peters PE, et al. Parasternal mediastinal sonography: sensitivity in the detection of anterior mediastinal and subcarinal tumors. AJR Am J Roentgenol 1988;150:1021-6. [PubMed]
- Wernecke K, Vassallo P, Peters PE, et al. Mediastinal tumors: biopsy under US guidance. Radiology 1989;172:473-6. [PubMed]
- Wernecke K, Vassallo P, Peters PE, et al. Diagnostic imaging of mediastinal tumors. Sensitivity and specificity of sonography in comparison with computed tomography and conventional x- ray diagnosis. Radiologe 1990;30:532-40. [PubMed]
- Wernecke K, Vassallo P, Potter R, et al. Mediastinal tumors: sensitivity of detection with sonography compared with CT and radiography. Radiology 1990;175:137-43. [PubMed]
- Wernecke K, Diederich S. Sonographic features of mediastinal tumors. AJR Am J Roentgenol 1994;163:1357-64. [PubMed]
- Hirche TO, Wagner TO, Dietrich CF. Mediastinal ultrasound: technique and possible applications. Med Klin (Munich) 2002;97:472-9. [PubMed]
- Goeckenjan G, Sitter H, Thomas M, et al. Prevention, diagnosis, therapy, and follow-up of lung cancer. Interdisciplinary guideline of the German Respiratory Society and the German Cancer Society--abridged version. Pneumologie 2011;65:e51-75. [PubMed]
- Dietrich CF, Ponnudurai R, Bachmann Nielsen M. Is there a need for new imaging methods for lymph node evaluation? Ultraschall Med 2012;33:411-4. [PubMed]
- Fujiwara T, Yasufuku K, Nakajima T, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest 2010;138:641-7. [PubMed]
- Dietrich CF, Averkiou MA, Correas JM, et al. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med 2012;33:344-51. [PubMed]
- Piscaglia F, Nolsoe C, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 2012;33:33-59. [PubMed]
- Nakajima T, Anayama T, Shingyoji M, et al. Vascular image patterns of lymph nodes for the prediction of metastatic disease during EBUS-TBNA for mediastinal staging of lung cancer. J Thorac Oncol 2012;7:1009-14. [PubMed]
- Nakajima T, Anayama T, Koike T, et al. Endobronchial ultrasound doppler image features correlate with mRNA expression of HIF1-alpha and VEGF-C in patients with non-small-cell lung cancer. J Thorac Oncol 2012;7:1661-7. [PubMed]
- Hocke M, Menges M, Topalidis T, et al. Contrast-enhanced endoscopic ultrasound in discrimination between benign and malignant mediastinal and abdominal lymph nodes. J Cancer Res Clin Oncol 2008;134:473-80. [PubMed]
- Dietrich CF. Elastography, the new dimension in ultrasonography. Praxis (Bern 1994) 2011;100:1533-42.
- Dietrich CF. Real-time tissue elastography. Multiple clinical applications. Multiple clinical solutions. Endoskopie heute 2012;24:177-212.
- Janssen J, Dietrich CF, Will U, et al. Endosonographic elastography in the diagnosis of mediastinal lymph nodes. Endoscopy 2007;39:952-7. [PubMed]
- Wiersema MJ, Hassig WM, Hawes RH, et al. Mediastinal lymph node detection with endosonography. Gastrointest Endosc 1993;39:788-93. [PubMed]
- Kalaitzakis E, Sadik R, Doig L, et al. Defining the lymph node burden in a Northern European population without malignancy: the potential effect of geography in determining a need for FNA? Dis Esophagus 2009;22:409-17. [PubMed]
- Catalano MF, Sivak MV Jr, Rice T, et al. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc 1994;40:442-6. [PubMed]
- Memoli JS, El Bayoumi E, Pastis NJ, et al. Using endobronchial ultrasound features to predict lymph node metastasis in patients with lung cancer. Chest 2011;140:1550-6. [PubMed]
- Roberts SA, Mahon BS, Evans R. Coagulation necrosis in malignant mediastinal nodes on endoscopic ultrasound: a new endosonographic sign. Clin Radiol 2005;60:587-91. [PubMed]
- Bhutani MS, Saftoiu A, Chaya C, et al. Irregular echogenic foci representing coagulation necrosis: a useful but perhaps under-recognized EUS echo feature of malignant lymph node invasion. J Gastrointestin Liver Dis 2009;18:181-4. [PubMed]
- Sawhney MS, Debold SM, Kratzke RA, et al. Central intranodal blood vessel: a new EUS sign described in mediastinal lymph nodes. Gastrointest Endosc 2007;65:602-8. [PubMed]
- Schmid-Bindert G, Jiang H, Kahler G, et al. Predicting malignancy in mediastinal lymph nodes by endobronchial ultrasound: a new ultrasound scoring system. Respirology 2012;17:1190-8. [PubMed]
- Faige DO. EUS in patients with benign and malignant lymphadenopathy. Gastrointest Endosc 2001;53:593-8. [PubMed]
- Chen VK, Eloubeidi MA. Endoscopic ultrasound-guided fine needle aspiration is superior to lymph node echofeatures: a prospective evaluation of mediastinal and peri-intestinal lymphadenopathy. Am J Gastroenterol 2004;99:628-33. [PubMed]
- Gill KR, Ghabril MS, Jamil LH, et al. Endosonographic features predictive of malignancy in mediastinal lymph nodes in patients with lung cancer. Gastrointest Endosc 2010;72:265-71. [PubMed]
- Schmulewitz N, Wildi SM, Varadarajulu S, et al. Accuracy of EUS criteria and primary tumor site for identification of mediastinal lymph node metastasis from non-small-cell lung cancer. Gastrointest Endosc 2004;59:205-12. [PubMed]
- Dietrich CF. Kursbuch Endosonografie. Stuttgart, New York: Thieme Georg Verlag, 2013.
- Holty JE, Kuschner WG, Gould MK. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-analysis. Thorax 2005;60:949-55. [PubMed]
- Herth FJ, Becker HD, Ernst A. Ultrasound-guided transbronchial needle aspiration: an experience in 242 patients. Chest 2003;123:604-7. [PubMed]
- Herth FJ, Rabe KF, Gasparini S, et al. Transbronchial and transoesophageal (ultrasound-guided) needle aspirations for the analysis of mediastinal lesions. Eur Respir J 2006;28:1264-75. [PubMed]
- Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest 2004;125:322-5. [PubMed]
- Rintoul RC, Skwarski KM, Murchison JT, et al. Endoscopic and endobronchial ultrasound real-time fine-needle aspiration for staging of the mediastinum in lung cancer. Chest 2004;126:2020-2. [PubMed]
- Rintoul RC, Skwarski KM, Murchison JT, et al. Endobronchial and endoscopic ultrasound-guided real-time fine-needle aspiration for mediastinal staging. Eur Respir J 2005;25:416-21. [PubMed]
- Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006;130:710-8. [PubMed]
- Herth FJ, Lunn W, Eberhardt R, et al. Transbronchial versus transesophageal ultrasound-guided aspiration of enlarged mediastinal lymph nodes. Am J Respir Crit Care Med 2005;171:1164-7. [PubMed]
- Herth FJ, Eberhardt R, Vilmann P, et al. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax 2006;61:795-8. [PubMed]
- Adams K, Shah PL, Edmonds L, et al. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009;64:757-62. [PubMed]
- Dong X, Qiu X, Liu Q, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the mediastinal staging of non-small cell lung cancer: a meta-analysis. Ann Thorac Surg 2013;96:1502-7. [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [PubMed]
- Zhang R, Ying K, Shi L, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: a meta-analysis. Eur J Cancer 2013;49:1860-7. [PubMed]
- Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest 2007;131:539-48. [PubMed]
- Vilmann P, Krasnik M, Larsen SS, et al. Transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: a combined approach in the evaluation of mediastinal lesions. Endoscopy 2005;37:833-9. [PubMed]
- Schröder C, Schönhofer B, Vogel B. Transesophageal echographic determination of aortic invasion by lung cancer. Chest 2005;127:438-42. [PubMed]
- Annema JT, Veselic M, Rabe KF. EUS-guided FNA of centrally located lung tumours following a non-diagnostic bronchoscopy. Lung Cancer 2005;48:357-61. [PubMed]
- Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S-20S.
- Annema JT, Versteegh MI, Veselic M, et al. Endoscopic ultrasound added to mediastinoscopy for preoperative staging of patients with lung cancer. JAMA 2005;294:931-6. [PubMed]
- Dhooria S, Aggarwal AN, Gupta D, et al. Utility and Safety of Endoscopic Ultrasound With Bronchoscope-Guided Fine-Needle Aspiration in Mediastinal Lymph Node Sampling: Systematic Review and Meta-Analysis. Respir Care 2015;60:1040-50. [PubMed]
- Annema JT, Versteegh MI, Veselic M, et al. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of lung cancer and its impact on surgical staging. J Clin Oncol 2005;23:8357-61. [PubMed]
- Tournoy KG, de Ryck F, Vanwalleghem LR, et al. Endoscopic ultrasound reduces surgical mediastinal staging in lung cancer: a randomized trial. Am J Respir Crit Care Med 2008;177:531-5. [PubMed]
- Dooms C, Tournoy KG, Schuurbiers O, et al. Endosonography for mediastinal nodal staging of clinical N1 non-small cell lung cancer: a prospective multicenter study. Chest 2015;147:209-15. [PubMed]
- Wallace MB, Ravenel J, Block MI, et al. Endoscopic ultrasound in lung cancer patients with a normal mediastinum on computed tomography. Ann Thorac Surg 2004;77:1763-8. [PubMed]
- Fernández-Esparrach G, Ginès A, Belda J, et al. Transesophageal ultrasound-guided fine needle aspiration improves mediastinal staging in patients with non-small cell lung cancer and normal mediastinum on computed tomography. Lung Cancer 2006;54:35-40. [PubMed]
- Herth FJ. Nonsurgical staging of the mediastinum: EBUS and EUS. Semin Respir Crit Care Med 2011;32:62-8. [PubMed]
- Herth FJ, Eberhardt R, Krasnik M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest 2008;133:887-91. [PubMed]
- Ge X, Guan W, Han F, et al. Comparison of Endobronchial Ultrasound-Guided Fine Needle Aspiration and Video-Assisted Mediastinoscopy for Mediastinal Staging of Lung Cancer. Lung 2015;193:757-66. [PubMed]
- Nakajima T, Yasufuku K, Fujiwara T, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of intrapulmonary lesions. J Thorac Oncol 2008;3:985-8. [PubMed]
- Tournoy KG, Rintoul RC, van Meerbeeck JP, et al. EBUS-TBNA for the diagnosis of central parenchymal lung lesions not visible at routine bronchoscopy. Lung Cancer 2009;63:45-9. [PubMed]
- Bugalho A, Ferreira D, Eberhardt R, et al. Diagnostic value of endobronchial and endoscopic ultrasound-guided fine needle aspiration for accessible lung cancer lesions after non-diagnostic conventional techniques: a prospective study. BMC Cancer 2013;13:130. [PubMed]
- Dietrich CF, Wehrmann T, Hoffmann C, et al. Detection of the adrenal glands by endoscopic or transabdominal ultrasound. Endoscopy 1997;29:859-64. [PubMed]
- Goerg C, Schwerk WB, Wolf M, et al. Adrenal masses in lung cancer: sonographic diagnosis and follow-up. Eur J Cancer 1992;28A:1400-3. [PubMed]
- Jenssen C, Dietrich CF. Ultrasound and endoscopic ultrasound of the adrenal glands. Ultraschall Med 2010;31:228-47. [PubMed]
- Eloubeidi MA, Black KR, Tamhane A, et al. A large single-center experience of EUS-guided FNA of the left and right adrenal glands: diagnostic utility and impact on patient management. Gastrointest Endosc 2010;71:745-53. [PubMed]
- Tuma J, Bock A, Dietrich CF. Nieren, Nebennieren. In: Tuma J, Trinkler FB, editors. Sonographische Differenzialdiagnose. Krankheiten des Urogenitalsystems. Systematischer Atlas. Köln: Deutscher Ärzte-Verlag, 2009;27-229.
- Schuurbiers OC, Tournoy KG, Schoppers HJ, et al. EUS-FNA for the detection of left adrenal metastasis in patients with lung cancer. Lung Cancer 2011;73:310-5. [PubMed]
- Dietrich CF, Wehrmann T, Caspary WF, et al. Häufigkeit und Bedeutung der Darstellung der linken Nebenniere mittels endoskopischer Ultraschalltechnik. Ultraschall in Med 1997;18:S34. (Abstract).
- Dietrich CF. Nebenniere. In: Dietrich CF, editor. Ultraschall-Kurs. Köln: Deutscher Ärzteverlag, 2006:249-58.
- Eloubeidi MA, Seewald S, Tamhane A, et al. EUS-guided FNA of the left adrenal gland in patients with thoracic or GI malignancies. Gastrointest Endosc 2004;59:627-33. [PubMed]
- Jhala NC, Jhala D, Eloubeidi MA, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy of the adrenal glands: analysis of 24 patients. Cancer 2004;102:308-14. [PubMed]
- Trojan J, Schwarz W, Sarrazin C, et al. Role of ultrasonography in the detection of small adrenal masses. Ultraschall Med 2002;23:96-100. [PubMed]
- Dietrich CF, Ignee A, Barreiros AP, et al. Contrast-enhanced ultrasound for imaging of adrenal masses. Ultraschall Med 2010;31:163-8. [PubMed]
- Dietrich CF, Hocke M, Jenssen C. Interventional endosonography. Ultraschall Med 2011;32:8-22. quiz. [PubMed]
- Eloubeidi MA, Morgan DE, Cerfolio RJ, et al. Transduodenal EUS-guided FNA of the right adrenal gland. Gastrointest Endosc 2008;67:522-7. [PubMed]
- DeWitt JM. Endoscopic ultrasound-guided fine-needle aspiration of right adrenal masses: report of 2 cases. J Ultrasound Med 2008;27:261-7. [PubMed]
- Uemura S, Yasuda I, Kato T, et al. Preoperative routine evaluation of bilateral adrenal glands by endoscopic ultrasound and fine-needle aspiration in patients with potentially resectable lung cancer. Endoscopy 2013;45:195-201. [PubMed]
- DeWitt J, LeBlanc J, McHenry L, et al. Endoscopic ultrasound-guided fine needle aspiration cytology of solid liver lesions: a large single-center experience. Am J Gastroenterol 2003;98:1976-81. [PubMed]
- Crowe DR, Eloubeidi MA, Chhieng DC, et al. Fine-needle aspiration biopsy of hepatic lesions: computerized tomographic-guided versus endoscopic ultrasound-guided FNA. Cancer 2006;108:180-5. [PubMed]
- Ardengh JC, Lopes CV, Kemp R, et al. Accuracy of endoscopic ultrasound-guided fine-needle aspiration in the suspicion of pancreatic metastases. BMC Gastroenterol 2013;13:63. [PubMed]
- El Hajj II, LeBlanc JK, Sherman S, et al. Endoscopic ultrasound-guided biopsy of pancreatic metastases: a large single-center experience. Pancreas 2013;42:524-30. [PubMed]
- Atiq M, Bhutani MS, Ross WA, et al. Role of endoscopic ultrasonography in evaluation of metastatic lesions to the pancreas: a tertiary cancer center experience. Pancreas 2013;42:516-23. [PubMed]
- DeWitt J, Jowell P, LeBlanc J, et al. EUS-guided FNA of pancreatic metastases: a multicenter experience. Gastrointest Endosc 2005;61:689-96. [PubMed]
- Hahn M, Faigel DO. Frequency of mediastinal lymph node metastases in patients undergoing EUS evaluation of pancreaticobiliary masses. Gastrointest Endosc 2001;54:331-5. [PubMed]
- Fritscher-Ravens A, Sriram PV, Bobrowski C, et al. Mediastinal lymphadenopathy in patients with or without previous malignancy: EUS-FNA-based differential cytodiagnosis in 153 patients. Am J Gastroenterol 2000;95:2278-84. [PubMed]
- Navani N, Nankivell M, Woolhouse I, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of intrathoracic lymphadenopathy in patients with extrathoracic malignancy: a multicenter study. J Thorac Oncol 2011;6:1505-9. [PubMed]
- Ardengh JC, Bammann RH, Giovani M, et al. Endoscopic ultrasound-guided biopsies for mediastinal lesions and lymph node diagnosis and staging. Clinics (Sao Paulo) 2011;66:1579-83. [PubMed]
- Peric R, Schuurbiers OC, Veselic M, et al. Transesophageal endoscopic ultrasound-guided fine-needle aspiration for the mediastinal staging of extrathoracic tumors: a new perspective. Ann Oncol 2010;21:1468-71. [PubMed]
- Tournoy KG, Govaerts E, Malfait T, et al. Endobronchial ultrasound-guided transbronchial needle biopsy for M1 staging of extrathoracic malignancies. Ann Oncol 2011;22:127-31. [PubMed]
- Ozgül MA, Cetinkaya E, Tutar N, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of intrathoracic lymphadenopathy in patients with extrathoracic malignancy: A study in a tuberculosis-endemic country. J Cancer Res Ther 2013;9:416-21. [PubMed]
- Şentürk A, Kiliç H, Hezer H, et al. Endobronchial ultrasound-guided transbronchial needle biopsy for the diagnosis of mediastinal lymphadenopathy in patients with extrathoracic malignancies. Turk J Med Sci 2014;44:989-95. [PubMed]
- Parmaksız ET, Caglayan B, Salepci B, et al. The utility of endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal or hilar lymph node evaluation in extrathoracic malignancy: Benign or malignant? Ann Thorac Med 2012;7:210-4. [PubMed]
- Agarwal B, Gogia S, Eloubeidi MA, et al. Malignant mediastinal lymphadenopathy detected by staging EUS in patients with pancreaticobiliary cancer. Gastrointest Endosc 2005;61:849-53. [PubMed]
- Sobel JM, Lai R, Mallery S, et al. The utility of EUS-guided FNA in the diagnosis of metastatic breast cancer to the esophagus and the mediastinum. Gastrointest Endosc 2005;61:416-20. [PubMed]
- Mortensen MB, Pless T, Durup J, et al. Clinical impact of endoscopic ultrasound-guided fine needle aspiration biopsy in patients with upper gastrointestinal tract malignancies. A prospective study. Endoscopy 2001;33:478-83. [PubMed]
- Hassan H, Vilmann P, Sharma V. Impact of EUS-guided FNA on management of gastric carcinoma. Gastrointest Endosc 2010;71:500-4. [PubMed]
- Wildi SM, Fickling WE, Day TA, et al. Endoscopic ultrasonography in the diagnosis and staging of neoplasms of the head and neck. Endoscopy 2004;36:624-30. [PubMed]
- Giovannini M, Bernardini D, Seitz JF, et al. Value of endoscopic ultrasonography for assessment of patients presenting elevated tumor marker levels after surgery for colorectal cancers. Endoscopy 1998;30:469-76. [PubMed]
- Paquin SC. Training in endoscopic ultrasound-guided fine needle aspiration. Endosc Ultrasound 2014;3:12-6. [PubMed]
- Sahai AV. Endoscopic ultrasound-guided fine-needle aspiration studies: Fanning the flames. Endosc Ultrasound 2014;3:68-70. [PubMed]
- Sahai AV. Endoscopic ultrasound-guided fine-needle aspiration: Getting to the point. Endosc Ultrasound 2014;3:1-2. [PubMed]
- Wani S. Basic techniques in endoscopic ultrasound-guided fine-needle aspiration: Role of a stylet and suction. Endosc Ultrasound 2014;3:17-21. [PubMed]
- Konge L, Annema J, Vilmann P, et al. Transesophageal ultrasonography for lung cancer staging: learning curves of pulmonologists. J Thorac Oncol 2013;8:1402-8. [PubMed]
- Konge L, Annema J. Assessment of endobronchial ultrasound-guided transbronchial needle aspiration performance. Am J Respir Crit Care Med 2013;188:254. [PubMed]
- Konge L, Vilmann P, Clementsen P, et al. Reliable and valid assessment of competence in endoscopic ultrasonography and fine-needle aspiration for mediastinal staging of non-small cell lung cancer. Endoscopy 2012;44:928-33. [PubMed]
- Yang B, Li F, Shi W, et al. Endobronchial ultrasound-guided transbronchial needle biopsy for the diagnosis of intrathoracic lymph node metastases from extrathoracic malignancies: a meta-analysis and systematic review. Respirology 2014;19:834-41. [PubMed]
- Konge L, Annema J, Clementsen P, et al. Using virtual-reality simulation to assess performance in endobronchial ultrasound. Respiration 2013;86:59-65. [PubMed]
- Stather DR, Maceachern P, Rimmer K, et al. Validation of an endobronchial ultrasound simulator: differentiating operator skill level. Respiration 2011;81:325-32. [PubMed]
- Sharma M, Rai P, Mehta V, et al. Techniques of imaging of the aorta and its first order branches by endoscopic ultrasound (with videos). Endosc Ultrasound 2015;4:98-108. [PubMed]
- Baron TH. Endoscopic ultrasound training in mid-to-late career: Falling prey to the dark side or the bright side? Endosc Ultrasound 2014;3:200-1. [PubMed]
- Bhutani MS. Endoscopic ultrasound comes of age: Mature, established, creative and here to stay! Endosc Ultrasound 2014;3:143-51. [PubMed]
- Dincer HE, Gliksberg EP, Andrade RS. Endoscopic ultrasound and/or endobronchial ultrasound-guided needle biopsy of central intraparenchymal lung lesions not adjacent to airways or esophagus. Endosc Ultrasound 2015;4:40-3. [PubMed]
- Fuccio L, Larghi A. Endoscopic ultrasound-guided fine needle aspiration: How to obtain a core biopsy? Endosc Ultrasound 2014;3:71-81. [PubMed]
- Harris K, Maroun R, Attwood K, et al. Comparison of cytologic accuracy of endobronchial ultrasound transbronchial needle aspiration using needle suction versus no suction. Endosc Ultrasound 2015;4:115-9. [PubMed]
- Hébert-Magee S. Basic technique for solid lesions: Cytology, core, or both? Endosc Ultrasound 2014;3:28-34. [PubMed]
- Lachter J. Basic technique in endoscopic ultrasound-guided fine needle aspiration for solid lesions: What needle is the best? Endosc Ultrasound 2014;3:46-53. [PubMed]
- Davoudi M, Colt HG, Osann KE, et al. Endobronchial ultrasound skills and tasks assessment tool: assessing the validity evidence for a test of endobronchial ultrasound-guided transbronchial needle aspiration operator skill. Am J Respir Crit Care Med 2012;186:773-9. [PubMed]
- Stather DR, Maceachern P, Rimmer K, et al. Assessment and learning curve evaluation of endobronchial ultrasound skills following simulation and clinical training. Respirology 2011;16:698-704. [PubMed]
- Wahidi MM, Hulett C, Pastis N, et al. Learning experience of linear endobronchial ultrasound among pulmonary trainees. Chest 2014;145:574-8. [PubMed]
- Stather DR, Chee A, Maceachern P, et al. Endobronchial ultrasound learning curve in interventional pulmonary fellows. Respirology 2015;20:333-9. [PubMed]
- Konge L, Colella S, Vilmann P, et al. How to learn and to perform endoscopic ultrasound and endobronchial ultrasound for lung cancer staging: A structured guide and review. Endosc Ultrasound 2015;4:4-9. [PubMed]
- Felip E, Garrido P, Trigo JM, et al. SEOM guidelines for the management of non-small-cell lung cancer (NSCLC). Clin Transl Oncol 2009;11:284-9. [PubMed]
- Wernecke K, Vassallo P, Hoffmann G, et al. Value of sonography in monitoring the therapeutic response of mediastinal lymphoma: comparison with chest radiography and CT. AJR Am J Roentgenol 1991;156:265-72. [PubMed]
- Wernecke K, Vassallo P, Rutsch F, et al. Thymic involvement in Hodgkin disease: CT and sonographic findings. Radiology 1991;181:375-83. [PubMed]
- Ko HM, da Cunha SG, Darling G, et al. Diagnosis and subclassification of lymphomas and non-neoplastic lesions involving mediastinal lymph nodes using endobronchial ultrasound-guided transbronchial needle aspiration. Diagn Cytopathol 2013;41:1023-30. [PubMed]
- Moonim MT, Breen R, Fields PA, et al. Diagnosis and subtyping of de novo and relapsed mediastinal lymphomas by endobronchial ultrasound needle aspiration. Am J Respir Crit Care Med 2013;188:1216-23. [PubMed]
- Nunez AL, Jhala NC, Carroll AJ, et al. Endoscopic ultrasound and endobronchial ultrasound-guided fine-needle aspiration of deep-seated lymphadenopathy: Analysis of 1338 cases. Cytojournal 2012;9:14. [PubMed]
- Yasuda I, Goto N, Tsurumi H, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy for diagnosis of lymphoproliferative disorders: feasibility of immunohistological, flow cytometric, and cytogenetic assessments. Am J Gastroenterol 2012;107:397-404. [PubMed]
- Grosu HB, Iliesiu M, Caraway NP, et al. Endobronchial Ultrasound Guided Transbronchial Needle Aspiration Accurately Diagnoses and Subtypes Lymphoma. Ann Am Thorac Soc 2015;12:1336-44. [PubMed]
- Jenssen C, Dietrich CF. Kontraindikationen, Komplikationen, Komplikationsmanagment. In: Dietrich CF, Nurnberg D, editors. Interventionelle Sonographie. Stuttgart: Thieme Verlag, 2011:127-60.
- Agarwal R, Srinivasan A, Aggarwal AN, et al. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: a systematic review and meta-analysis. Respir Med 2012;106:883-92. [PubMed]
- Hirche TO, Hirche H, Cui XW, et al. Ultrasound evaluation of mediastinal lymphadenopathy in patients with sarcoidosis. Med Ultrason 2014;16:194-200. [PubMed]
- Sun J, Teng J, Yang H, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in diagnosing intrathoracic tuberculosis. Ann Thorac Surg 2013;96:2021-7. [PubMed]
- Puri R, Vilmann P, Sud R, et al. Endoscopic ultrasound-guided fine-needle aspiration cytology in the evaluation of suspected tuberculosis in patients with isolated mediastinal lymphadenopathy. Endoscopy 2010;42:462-7. [PubMed]
- Cui XW, Jenssen C, Saftoiu A, et al. New ultrasound techniques for lymph node evaluation. World J Gastroenterol 2013;19:4850-60. [PubMed]
- Popescu A, Saftoiu A. Can elastography replace fine needle aspiration? Endosc Ultrasound 2014;3:109-17. [PubMed]
- Iglesias-Garcia J, Lindkvist B, Larino-Noia J, et al. Endoscopic ultrasound elastography. Endosc Ultrasound 2012;1:8-16. [PubMed]
- von Bartheld MB, Veselic-Charvat M, Rabe KF, et al. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of sarcoidosis. Endoscopy 2010;42:213-7. [PubMed]
- Navani N, Booth HL, Kocjan G, et al. Combination of endobronchial ultrasound-guided transbronchial needle aspiration with standard bronchoscopic techniques for the diagnosis of stage I and stage II pulmonary sarcoidosis. Respirology 2011;16:467-72. [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Prospective study of endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes versus transbronchial lung biopsy of lung tissue for diagnosis of sarcoidosis. J Thorac Cardiovasc Surg 2012;143:1324-9. [PubMed]
- von Bartheld MB, Dekkers OM, Szlubowski A, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA 2013;309:2457-64. [PubMed]
- Trisolini R, Lazzari AL, Tinelli C, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for diagnosis of sarcoidosis in clinically unselected study populations. Respirology 2015;20:226-34. [PubMed]
- von Bartheld M. van der HE, Annema J. Mediastinal abscess formation after EUS-guided FNA: are patients with sarcoidosis at increased risk? Gastrointest Endosc 2012;75:1104-7. [PubMed]
- Fritscher-Ravens A, Ghanbari A, Topalidis T, et al. Granulomatous mediastinal adenopathy: can endoscopic ultrasound-guided fine-needle aspiration differentiate between tuberculosis and sarcoidosis? Endoscopy 2011;43:955-61. [PubMed]
- Navani N, Molyneaux PL, Breen RA, et al. Utility of endobronchial ultrasound-guided transbronchial needle aspiration in patients with tuberculous intrathoracic lymphadenopathy: a multicentre study. Thorax 2011;66:889-93. [PubMed]
- Geake J, Hammerschlag G, Nguyen P, et al. Utility of EBUS-TBNA for diagnosis of mediastinal tuberculous lymphadenitis: a multicentre Australian experience. J Thorac Dis 2015;7:439-48. [PubMed]
- Barreiros AP, Braden B, Schieferstein-Knauer C, et al. Characteristics of intestinal tuberculosis in ultrasonographic techniques. Scand J Gastroenterol 2008;43:1224-31. [PubMed]
- Jenssen C, Möller K, Sarbia M, et al. Endoscopic ultrasound-guided biopsy - indications, problems, pitfalls, troubleshooting, clinical impact. In: Dietrich CF, editor. Endoscopic ultrasound - an introductory manual and atlas. Stuttgart, New York: Thieme, 2011.
- Kennedy MP, Jimenez CA, Mhatre AD, et al. Clinical implications of granulomatous inflammation detected by endobronchial ultrasound transbronchial needle aspiration in patients with suspected cancer recurrence in the mediastinum. J Cardiothorac Surg 2008;3:8. [PubMed]
- DePew ZS, Gonsalves WI, Roden AC, et al. Granulomatous inflammation detected by endobronchial ultrasound-guided transbronchial needle aspiration in patients with a concurrent diagnosis of cancer: a clinical conundrum. J Bronchology Interv Pulmonol 2012;19:176-81. [PubMed]
- Dietrich CF, Chichakli M, Hirche TO, et al. Sonographic findings of the hepatobiliary-pancreatic system in adult patients with cystic fibrosis. J Ultrasound Med 2002;21:409-16. [PubMed]
- Dietrich CF, Chichakli M, Bargon J, et al. Mediastinal lymph nodes demonstrated by mediastinal sonography: activity marker in patients with cystic fibrosis. J Clin Ultrasound 1999;27:9-14. [PubMed]
- Dietrich CF, Stryjek-Kaminska D, Teuber G, et al. Perihepatic lymph nodes as a marker of antiviral response in patients with chronic hepatitis C infection. AJR Am J Roentgenol 2000;174:699-704. [PubMed]
- Dietrich CF, Leuschner MS, Zeuzem S, et al. Peri-hepatic lymphadenopathy in primary biliary cirrhosis reflects progression of the disease. Eur J Gastroenterol Hepatol 1999;11:747-53. [PubMed]
- Dietrich CF, Lee JH, Herrmann G, et al. Enlargement of perihepatic lymph nodes in relation to liver histology and viremia in patients with chronic hepatitis C. Hepatology 1997;26:467-72. [PubMed]
- Dietrich CF, Viel K, Braden B, et al. Mediastinal lymphadenopathy: an extrahepatic manifestation of chronic hepatitis C? Z Gastroenterol 2000;38:143-52. [PubMed]


