Asiatic acid inhibits cardiac hypertrophy by blocking interleukin-1β-activated nuclear factor-κB signaling in vitro and in vivo
Introduction
Cardiac hypertrophy can be categorized into physiological hypertrophy and pathological hypertrophy (1). The former condition is induced by exercise training and adaptation to the special movement environment, accompanied by increased cardiac function (2). The latter, however, is usually developed as a response to pathological conditions such as valvular heart disease, hypertension, etc., and its main manifestations include thickened ventricular wall and increased heart weight (HW) and cardiac myocyte volume (3,4). In its early phase, pathological hypertrophy develops as a compensatory response that increases cardiac output and subsequently causes conditions such as cardiac dysfunction, energy metabolism impairment and electrophysiological damage. These conditions deteriorates in later stages and eventually could lead to heart failure, arrhythmia and sudden death (5,6). Up to now, the molecular mechanisms contributing to the development of cardiac hypertrophy are incompletely understood.
Cardiac hypertrophy is developed as a result of extracellular stimuli activating nuclear genes via intracellular signaling pathway (7,8). A number of studies demonstrated that multiple signaling pathways could be contributing to the development of cardiac hypertrophy including mitogen-activated protein kinase (MAPK) pathway (9) and phosphoinositide 3-kinase (PI3K)/Akt pathway (10). Moreover, recent studies have identified interleukin (IL)-1β-activated nuclear factor (NF)-κB signaling plays a pivotal role in inducing cardiac hypertrophy, and its inhibition could block downstream pathways and prevent cardiac hypertrophy (11-13).
Asiatic acid (AA) is one of the triterpenoid compounds found in the centella asiatica. Many studies suggest that AA exhibits a variety of pharmacological effects, including anti-inflammatory (14), anti-hepatofibric (15), and neuroprotection benefits (16). Moreover, Huang et al. (17) recently reported that AA decreased the paw edema and inhibited inflammatory response in a mice model of paw edema, and the anti-inflammatory mechanisms of AA might be associated with the reduce in the level of IL-1β and NF-κB in the edema paw. In particular, it has been demonstrated that IL-1β is a potent modulator of cardiac hypertrophy, both in vitro and in vivo, through activating NF-κB signaling (12,13,18). Therefore, we hypothesized that AA may prevent cardiac hypertrophy by blocking IL-1β-activated NF-κB signaling. To test this hypothesis, an IL-1β-stimulated hypertrophic cardiomyocyte model and a pressure overload-induced cardiac hypertrophy model were used in the present study.
Materials and methods
Reagents
Purified natural AA (97%) and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Recombinant rat IL-1β was obtained from PeproTech (Rocky Hill, NJ, USA). Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco Corporation (Life Technologies, CA, USA). Cell Counter Kit-8 (CCK-8) assay kit was obtained from Dojindo Laboratories (Kumamoto, Japan). Electrophoretic mobility shift assay (EMSA) kit was obtained from Pierce Biotechnology (IL, USA). Cytoplasmic and nuclear protein extraction kits were obtained from Keygen Biotechnology (Nanjing, China). Unless otherwise indicated, chemicals and materials used in this study were purchased from Sigma.
Ethics statements
Animal handling and use is compliant with the “Guide for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (NIH Publication No.85-23, revised 1996) and were approved by the Animal Care and Use Committee of Nanjing Medical University. The animals had free access to standard food and water and were housed on a 12 h light/dark cycle at 22 °C room temperature. The experiments were designed to minimize pain and the number of animals used.
Primary cultures of neonatal rat ventricular myocytes
Hearts were immediately removed from 1 to 2-day-old neonatal Sprague-Dawley rats anesthetized by diethyl ether under aseptic conditions and washed in Ca2+ and Mg2+ free phosphate-buffered saline (PBS). After the atria and aorta were discarded, the ventricles were minced and enzymatically digested with 0.1% collagenase type I (Sigma) and 0.125% trypsin (Gibco). The liberated cells were collected by centrifugation and incubated in 100-mm culture dishes (Corning) for 90 minutes at 37 °C in a humidified incubator with 5% CO2 air. Non-adherent cells were harvested as cardiomyocytes and seeded at a density 1×106 per well into 6-well culture plates (Corning). They were incubated in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin and bromodeoxyuridine (BrdU, 100 µM, Sigma). After 48 h, the culture medium was replaced with DMEM containing 1% FBS. After 24 h of serum starvation, the cells were incubated with AA for 24 h prior to IL-1β (10 ng/mL) stimulation for 24 h. Untreated cells served as the controls. AA was freshly prepared as a stock solution in DMSO and diluted with sterile double-distilled water [0.1% (v/v) DMSO]. IL-1β was dissolved in sterile double-distilled water. There were five experimental groups: (I) control, (II) AA, (III) IL-1β, (IV) IL-1β + vehicle (DMSO-saline) and (V) IL-1β + AA.
Cell viability assay
Cell viability was monitored using a CCK-8 assay according to the manufacturer’s instructions. In brief, the cardiomyocytes were initially cultured at a density of 1×104 cells/well in 96-well plates. The cells were then pre-treated with various concentrations of AA (2.5-30 µM) for 24 h. CCK-8 solution (10 µL) was then added to each culture well followed by incubation for 4 h at 37 °C. The absorbance at 450 nm was measured using a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA). All experiments were performed in triplicate, and cell viability was calculated as a percentage.
Immunofluorescence analysis of cardiomyocytes
The cardiomyocytes were cultured on coverslips. Following IL-1β (10 ng/mL) stimulation for 24 h in the presence or absence of AA for 24 h, the cells were washed with PBS, fixed with 4% paraformaldehyde for 20 min and permeablized with 0.1% Triton X-100/PBS for 10 min. After blocking with 5% bovine serum for 30 min, the size of the cells was determined by staining the membranes with specific anti-sarcomeric α-actinin antibody (Sigma-Aldrich) and visualized under an inverted fluorescence microscope (Nikon, Tokyo, Japan). The size of the cardiomyocytes was determined using ImageJ software (NIH, Bethesda, MD, USA).
Animal models of cardiac hypertrophy (aortic banding)
An animal model of pressure overload-induced cardiac hypertrophy was created by transverse aortic constriction (TAC) in male C57BL/6 mice [8-10 weeks of age, 20-30 g body weight (BW); Experimental Animal Center of Jiangsu Province, Nanjing, China]. To achieve constriction, a 7-0 suture was snugly tied twice around a blunt 27-gauge needle, which was positioned adjacent to the aorta between the right innominate and left carotid arteries and promptly removed following ligation. This produced a 60-70% constriction with an outer aortic diameter of approximately 0.4 mm. Acute and chronic mortality from the ligature procedure was <10%. Sham-operated controls consisted of age-matched littermates that underwent an identical surgical procedure, including the isolation of the aorta, only without placement of the ligature. Twenty-four hours after the operation, the mice subjected to TAC and the sham-operated mice were orally gavaged with AA 100 mg/kg/day or with the vehicle (DMSO-saline). After 2 weeks of TAC, the hearts were harvested and the ratio of heart weight to body weight (HW/BW) was calculated. The heart samples were frozen in liquid nitrogen and stored at −80 °C. AA was freshly prepared as a stock solution in DMSO and diluted with saline to yield a final AA concentration of 100 mg/kg BW [0.1% (v/v) DMSO] (based on our preliminary experiment). The vehicle control was administered a mixture of DMSO with saline [0.1% (v/v) DMSO]. There were five experimental groups:
- No oral gavage mice (sham group, n=10)
- AA oral gavage mice (sham + AA group, n=10)
- Saline oral gavage TAC mice (TAC group, n=10)
- Vehicle oral gavage TAC mice (TAC + vehicle group, n=10)
- AA oral gavage TAC mice (TAC + AA group, n=10)
Transthoracic echocardiography
All mice were anesthetized by a mixture of isoflurane (1.5%) and oxygen (0.5 L/min). Cardiac dimensions and functions were evaluated by echocardiography (Vevo 2100 equipped with a 30-MHz high-resolution phase array transducer, VisualSonics, Toronto, ON, Canada) after 2 weeks following TAC. The left ventricle (LV) was assessed in both parasternal long-axis and short-axis views. End-systole and end-diastole were defined as the phase in which the smallest and largest area of LV were obtained, respectively. Interventricular septum diastolic dimension (IVSD), left ventricular posterior wall thickness diastole (LVPWD), left ventricular end-systolic diameter (LVESD) and left ventricular end-diastolic diameter (LVEDD) were measured from the LV M-mode tracing with a sweep speed of 50 mm/s at the papillary muscle level. The percentage fractional shortening (%FS) was calculated using a standard formula: %FS = [(LVEDD − LVESD)/LVEDD] ×100. At each location, for each mouse, 6-10 beats were analyzed.
Nuclear proteins extraction and electrophoretic mobility shift assays (EMSA)
Nuclear proteins were isolated from LV samples and cultured cardiomyocytes, as described in a previous study (12). NF-κB binding activity was examined using an EMSA kit (Pierce Biotechnology, Inc.), according to the manufacturer’s protocols. For the competition assay, specific unlabeled NF-κB competitors (200-fold molar excess) were employed along with the binding reaction mixture. In brief, 2 µL of nuclear extract (at a concentration of 2 µg/µL) was incubated with 1 µL biotin-labeled probe containing the NF-κB binding domain (5'-AGTTGAGGGGACTTTCCCAGGC-3', 3'-TCAACTCCCCTGAAAGGGTCCG-5') at room temperature for 20 min. The reaction mixture was separated on 6.5% polyacrylamide gel electrophoresis, and transferred onto nylon membranes. The membranes were subjected to ultraviolet (UV) light cross-link for 1 min, and then incubated with blocking buffer containing stabilized streptavidin-horseradish peroxidase conjugate (1:2,000) for 15 min. The signals on the membranes were detected with the Chemiluminesent Nucleic Acid Detection Module (Pierce Biotechnology, Inc.). The NF-κB binding bands were scanned by G:BOX-CHEMI-XR5-E (Syngene, Frederick, MD, USA) and the relative intensities were analyzed with ImageJ software (NIH).
Quantitative real time-polymerase chain reaction (RT-PCR)
mRNA transcripts were quantified by quantitative real time polymerase chain reaction (RT-qPCR). Briefly, RNAs from LV tissues and cardiomyocytes were isolated with RNAiso Trizol (Invitrogen, Carlsbad, CA, USA), respectively. cDNA generated from 500 ng of total RNA was reverse transcribed using the PrimeScriptTM RT reagent kit (Takara Biotechnology, Shiga, Japan). Specific products were determined using Eppendorf Mastercycler ep realplex analysis software according to the instructions provided with SYBR® Premix Ex TaqTM II (Tli RNaseH Plus; Takara Biotechnology). The specific forward and reverse primers used were as follows: mouse ANP forward, 5'-CCAGCATGGGCTCCTTCTCCA-3' and reverse, 5'-CCGGAAGCTGTTGCAGCCTAGT-3'; mouse IL-1β forward, 5'-TCATTGTGGCTGTGGAGAAG-3' and reverse, 5'-AGGCCACAGGTATTTTGTCG-3'; mouse glyceraldehydes-3-phosphate dehydrogenase (GAPDH) forward, 5'-GGCATCGTGGAGGGA-3' and reverse, 5'-TGAGTTAGACTGAGTGAAGAG-3', rat ANP forward, 5'-GCTCGAGCAGATCGCAAAAG-3' and reverse, 5'-CACCACCTCTCAGTGGCAAT-3'; and rat GAPDH forward, 5'-ATGGGAAGCTGGTCATCAAC-3' and reverse, 5'-GTGGTTCACACCCATCACAA-3'. The expression levels of all transcripts were normalized to the housekeeping gene GAPDH in the same tissue. The relative mRNA expression was calculated as follows: mRNA expression =2−(ΔCT sample − ΔCT control).
Western blot analysis
Total protein were extracted from LV tissues and cultured cardiomyocytes, respectively, and assessed by western blot analysis and enhanced chemiluminescence. The proteins (20-30 µg) were separated by 10-15% SDS-PAGE and subsequently transferred onto polyvinylidene difluoride (PVDF) membranes using a Mini Trans-Blot electrophoresis transfer cell (Bio-Rad Laboratories). The membranes were incubated with appropriate primary antibodies against IL-1β and GAPDH, respectively. After extensive washings in TBST (10 mM Tris-HCl, 150 mM NaCl and 0.1%Tween 20, pH 7.6), the membranes was incubated with the appropriate horse radish peroxidase (HRP)-conjugated secondary antibodies. The signals were detected with an electrochemical-luminescence (ECL) Western Blot Detection Kit (Thermo Scientific, Rockford, IL, USA), and blot quantification was performed using densitometry with ImageJ software (NIH).
Histology
The hearts were fixed in 10% neutral formalin, dehydrated in 75%, 80%, 90% and 100% ethanol, transferred to xylene, embedded in paraffin and sectioned at a thickness of 4-5 µm, then stained with hematoxylin and eosin (H&E). The cardiomyocyte cross-section area (CSA) was measured using NIH ImageJ software (NIH). At least three different hearts with five separate fields of cells (total 50-70 cells for each heart), were quantified for cellular analysis.
Statistical analysis
Data are expressed as the means ± standard deviation (SD). The GraphPad Prism 5.01 (GraphPad Software, Inc., La Jolla, CA, USA) and PASW Statistics 18.0 (SPSS Inc., Fayetteville, NC, USA) packages were used. Differences among groups were tested by one-way analysis of variance (ANOVA). Comparisons between two groups were performed by unpaired two-tailed Student’s t test. If a P value was <0.05, the result was considered statistically significant.
Results
AA inhibited the IL-1β-induced hypertrophic response in cardiomyocytes
Effects of AA (Figure 1A) alone on cardiomyocytes viability were first evaluated through the CCK-8 assay. As shown in Figure 1B, there was no noticeable change in the viability of cardiomyocytes treated with 2.5-30 µM AA for 24 h, but high concentrations of AA (40 and 80 µM) significantly decreased cell viability to 73.87% (P<0.05) and 15.99% (P<0.001), respectively.
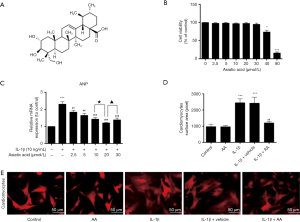
IL-1β has been reported to induce hypertrophic response in rat cardiomyocytes (19). To investigate whether AA can inhibit cardiomyocytes hypertrophy induced by IL-1β, the cells were treated with IL-1β (10 ng/mL) for 24 h in the presence or absence of AA (2.5-30 µM) for 24 h. The expression of ANP mRNA was examined by RT-qPCR. As shown in Figure 1C, IL-1β (10 ng/mL) stimulation remarkably increased the expression of ANP mRNA by 2.31 folds compared with the control group (P<0.001). AA (2.5-30 µM) pretreatment significantly decreased ANP mRNA expression, and the level of ANP mRNA in the AA (20 µM)-treated group was maximally reduced by 47.78% compared with IL-1β stimulated group (P<0.001).
In addition, the size of the cardiomyocytes was measured by immunofluorescence staining. As shown in Figure 1D and E, IL-1β stimulation induced a noticeable hypertrophic response in the cardiomyocytes that was not observed in the untreated control cells. In contrast, AA (20 µM) pretreatment attenuated the IL-1β-induced hypertrophic response of cardiomyocytes. However, the AA-treated cells were the same size as the untreated cells.
AA prevented the IL-1β-stimulated increase in NF-κB binding activity
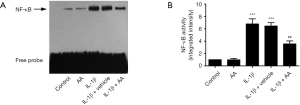
IL-1β stimulation plays a predominate role in the activation of NF-κB according to previously study (20). We examined the effect of AA on IL-1β-stimulated NF-κB binding activity. As shown in Figure 2, the NF-κB binding activity was dramatically increased after IL-1β (10 ng/mL) stimulation for 24 h compared with the untreated cells (P<0.001). In contrast, AA pretreatment substantially reduced the IL-1β-stimulated increase in NF-κB binding activity (P<0.01).
AA administration inhibited TAC-induced cardiac hypertrophy
The issue of whether AA could prevent pressure overload-induced cardiac hypertrophy was then addressed. The mice were subjected to TAC and treated with AA (100 mg/kg/daily) or with vehicle for 2 weeks. As shown in Figure 3A, pressure overload induced by TAC remarkably increased the ratio of HW/BW by 42.06% compared with the sham group (P<0.001). In contrast, AA administration effectively reduced the ratio of HW/BW by 28.27% compared with the TAC group (P<0.001).
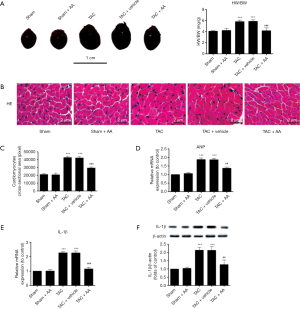
In addition, the heart sections were counterstained with H&E, pressure overload induced by TAC remarkably increased the cardiomyocytes CSA compared with the sham group (Figure 3B and C, P<0.001). In contrast, AA administration effectively reduced the TAC-induced increase in CSA (P<0.001).
ANP mRNA is considered as an indicator of hypertrophic remodeling (21). Figure 3D shows that pressure overload significantly increased the expression of ANP mRNA in the myocardium by 2.16 folds compared with the sham control (P<0.001). In contrast, AA administration prevented the TAC-induced increase in ANP mRNA expression (P<0.01). In addition, the expression of IL-1β mRNA and protein in the pressure-overloaded myocardium was markedly increased compared with the sham control (Figure 3E and F, P<0.001 and P<0.001 for IL-1β mRNA and protein, respectively). In contrast, AA administration substantially reduced the TAC-induced increases in the expression of IL-1β mRNA and protein in the myocardium (P<0.001 and P<0.001 for IL-1β mRNA and protein, respectively).
AA attenuated cardiac dysfunction induced by TAC
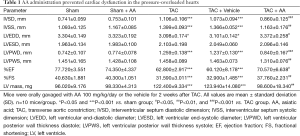
As shown in Table 1, TAC mice displayed a remarkable increase in IVSD and LVPWD compared with the sham operated mice (P<0.001 and P<0.001 for IVSD and LVPWD, respectively). In contrast, AA administration effectively reduced the TAC-induced increases in IVSD and LVPWD by 22.24% (P<0.001) and 33.28% (P<0.001), respectively. LVEDD was decreased after 2 weeks of pressure overload (P<0.05), and AA administration prevented the TAC-induced decrease in LVEDD (P<0.05). In addition, %FS was significantly decreased by 22.25% at 2 weeks after TAC compared with the sham group (P<0.001). In contrast, AA treatment attenuated the TAC-induced decrease in %FS (P<0.001).
Full table
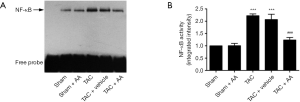
AA prevents the TAC-induced increase in NF-κB binding activity
Since activation of NF-κB has been demonstrated to play an important role in the development of cardiac hypertrophy induced by TAC (11). We then examined the effect of AA on TAC-induced NF-κB binding activity. As shown in Figure 4, the NF-κB binding activity was significantly increased in the pressure-overloaded myocardium compared with the sham group (P<0.001). In contrast, AA administration prevented TAC-induced increase in the NF-κB binding activity (P<0.001).
Discussion
In the present study, we demonstrated that (I) IL-1β-activated NF-κB signaling was involved in the development of cardiac hypertrophy both in vivo and in vitro. (II) AA prevented not only the cardiomyocyte hypertrophic response induced by IL-1β, but also the upregulation of IL-1β levels and cardiac hypertrophy induced by TAC. (III) The anti-hypertrophic mechanisms of AA were found to be associated with the reduction in IL-1β expression and the inhibition of NF-κB binding activity. These findings support the conclusion that AA could be a promising strategy for the prevention and treatment of cardiac hypertrophy.
IL-1β is a cytokine playing a key role in inflammation and is mainly generated by activated mononuclear-macrophage. It has been previously demonstrated that IL-1β and its activated signaling pathway are involved in the pathogenesis of many cardiovascular diseases, including coronary atherosclerosis, myocardial infarction and myocardial remodeling, as well as heart failure (22,23). Furthermore, significantly increased expression of IL-1β accompanied with the progressive LV hypertrophy was observed in the pressure overload-induced hypertensive rats (24,25). Accordingly, our in vitro experiments demonstrated that neonatal cardiac myocytes stimulated with IL-1β had obvious hypertrophic response, manifesting as enlarged cardiac myocyte size and increased expression of embryonic marker gene ANP. Our in vivo experiments further addressed the role of IL-1β in the development of cardiac hypertrophy. After 2 weeks of TAC procedure, markedly enlarged heart as well as increased LVPWD and IVSD were observed in the TAC mice. And the expression of IL-1β and ANP were also significantly increased in the mice undergone TAC surgery. Taken together, these data suggested that IL-1β can induce a hypertrophic response in the pressure-overloaded heart.
In recent years, many studies have confirmed that AA can block the stimulation of cytokines such as IL-1β, tumor necrosis factor alpha (TNF-α), transforming growth factor beta 1 (TGF-β1) and their related signaling pathways, and relieve functional damage and fibrosis of liver cells (15,26). However, there is no relevant report on whether AA can prevent cardiac hypertrophy in response to pressure overload. Fortunately, our study revealed that AA significantly inhibited IL-1β-induced hypertrophic response of cardiomyocytes as reflected by reduction in the cardiomyocyte surface area and the inhibition of ANP expression. In TAC model mice, AA administration significantly attenuated pressure overload-induced cardiac hypertrophy and improved cardiac performance by reducing the dimensions of the left ventricular chamber. Furthermore, the expression levels of IL-1β and ANP in the pressure-overloaded myocardium were also reduced after AA treatment. Therefore, our results may be the evidence of AA’s ability to blunt the cardiac pathological hypertrophy by inhibiting IL-1β related signaling pathways.
Cardiac hypertrophy is associated with multiple intracellular signaling pathways (7,8). Zhu et al. (12) confirmed that IL-1β was closely associated with cardiac hypertrophy by activating IL-1R-mediated MyD88-dependent signaling pathway in vitro and in vivo. After IL-1β binds its receptor (IL-1R), myeloid differentiation primary response gene 88 (MyD88) is recruited and facilitates the assembly of signaling complexes (20,27). Subsequently, NF-κB is activated and initiates the development of cardiac hypertrophy. It has been demonstrated that activated NF-κB plays a critical role in the production of inflammatory cytokines and is essential to the development of cardiac hypertrophy (11,12). Conversely, inhibition of NF-κB binding activity may be a means of preventing cardiac hypertrophy. For instance, the inactivation of NF-κB with direct gene transfection of sh-p65 RNA has been shown to result in the attenuation of cardiac hypertrophy (28). In addition, Li et al. (11) reported that blocking NF-κB binding activity in the pressure-overloaded myocardium significantly attenuated cardiac hypertrophy induced by TAC. Our observation is consistent with these previous reports showing that increased NF-κB binding activity was observed in an IL-1β-dependent manner. Surprisingly, AA treatment remarkably inhibited NF-κB binding activity as well as IL-1β expression in the pressure-overload hypertrophic heart, suggesting that IL-1β-dependent NF-κB signaling pathway may play an important role in cardiac hypertrophy.
Conclusions
In summary, AA inhibited cardiac hypertrophy in IL-1β stimulation experiment in vitro, and inhibited TAC-induced cardiac hypertrophy in vivo. Therefore, AA could potentially treat cardiac hypertrophy. Results of this study has highlighted the efficacy of AA in blocking IL-1β-activated NF-κB signaling, and it compels further investigation of more detailed mechanism of NF-κB deactivation, and its physiological effects as potential treatment of pathological cardiac hypertrophy.
Acknowledgements
Funding: This work was supported by grants from the Natural Science Foundation of Jiangsu Higher Education Institutions (12KJB320003); the Administration of Traditional Chinese Medicine of Jiangsu Province (lz13217); the Nanjing Foundation for Development of Science and Technology (201303036); the National Natural Science Foundation of China (81300128); Ph.D. Programs Foundation of Ministry of Education of China (20123234120015); the Jiangsu Natural Science Foundation (BK20131025); the Project Sponsored by the Scientific Research Foundation for Returned Overseas Chinese Scholars, State Education Ministry; and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Abel ED, Doenst T. Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res 2011;90:234-42. [PubMed]
- Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol 2013;14:38-48. [PubMed]
- Carabello BA. Aortic stenosis: from pressure overload to heart failure. Heart Fail Clin 2006;2:435-42. [PubMed]
- Papademetriou V. From hypertension to heart failure. J Clin Hypertens (Greenwich) 2004;6:14-7. [PubMed]
- van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest 2013;123:37-45. [PubMed]
- Oka T, Akazawa H, Naito AT, et al. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res 2014;114:565-71. [PubMed]
- Molkentin JD, Dorn GW 2nd. Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol 2001;63:391-426. [PubMed]
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 2006;7:589-600. [PubMed]
- Wang Y. Mitogen-activated protein kinases in heart development and diseases. Circulation 2007;116:1413-23. [PubMed]
- Dorn GW 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest 2005;115:527-37. [PubMed]
- Li Y, Ha T, Gao X, et al. NF-kappaB activation is required for the development of cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol 2004;287:H1712-20. [PubMed]
- Zhu Y, Li T, Song J, et al. The TIR/BB-loop mimetic AS-1 prevents cardiac hypertrophy by inhibiting IL-1R-mediated MyD88-dependent signaling. Basic Res Cardiol 2011;106:787-99. [PubMed]
- Higashikuni Y, Tanaka K, Kato M, et al. Toll-like receptor-2 mediates adaptive cardiac hypertrophy in response to pressure overload through interleukin-1β upregulation via nuclear factor κB activation. J Am Heart Assoc 2013;2:e000267. [PubMed]
- Yun KJ, Kim JY, Kim JB, et al. Inhibition of LPS-induced NO and PGE2 production by asiatic acid via NF-kappa B inactivation in RAW 264.7 macrophages: possible involvement of the IKK and MAPK pathways. Int Immunopharmacol 2008;8:431-41. [PubMed]
- Tang LX, He RH, Yang G, et al. Asiatic acid inhibits liver fibrosis by blocking TGF-beta/Smad signaling in vivo and in vitro. PLoS One 2012;7:e31350. [PubMed]
- Krishnamurthy RG, Senut MC, Zemke D, et al. Asiatic acid, a pentacyclic triterpene from Centella asiatica, is neuroprotective in a mouse model of focal cerebral ischemia. J Neurosci Res 2009;87:2541-50. [PubMed]
- Huang SS, Chiu CS, Chen HJ, et al. Antinociceptive activities and the mechanisms of anti-inflammation of asiatic Acid in mice. Evid Based Complement Alternat Med 2011;2011:895857.
- Petersen CA, Krumholz KA, Burleigh BA. Toll-like receptor 2 regulates interleukin-1beta-dependent cardiomyocyte hypertrophy triggered by Trypanosoma cruzi. Infect Immun 2005;73:6974-80. [PubMed]
- Palmer JN, Hartogensis WE, Patten M, et al. Interleukin-1 beta induces cardiac myocyte growth but inhibits cardiac fibroblast proliferation in culture. J Clin Invest 1995;95:2555-64. [PubMed]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 2004;4:499-511. [PubMed]
- Feng JA, Perry G, Mori T, et al. Pressure-independent enhancement of cardiac hypertrophy in atrial natriuretic peptide-deficient mice. Clin Exp Pharmacol Physiol 2003;30:343-9. [PubMed]
- Bujak M, Frangogiannis NG. The role of IL-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp (Warsz) 2009;57:165-76. [PubMed]
- Isoda K, Ohsuzu F. The effect of interleukin-1 receptor antagonist on arteries and cholesterol metabolism. J Atheroscler Thromb 2006;13:21-30. [PubMed]
- Shioi T, Matsumori A, Kihara Y, et al. Increased expression of interleukin-1 beta and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the hypertrophied and failing heart with pressure overload. Circ Res 1997;81:664-71. [PubMed]
- Honsho S, Nishikawa S, Amano K, et al. Pressure-mediated hypertrophy and mechanical stretch induces IL-1 release and subsequent IGF-1 generation to maintain compensative hypertrophy by affecting Akt and JNK pathways. Circ Res 2009;105:1149-58. [PubMed]
- Guo W, Liu W, Hong S, et al. Mitochondria-dependent apoptosis of con A-activated T lymphocytes induced by asiatic acid for preventing murine fulminant hepatitis. PLoS One 2012;7:e46018. [PubMed]
- Medzhitov R, Preston-Hurlburt P, Kopp E, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 1998;2:253-8. [PubMed]
- Gupta S, Young D, Maitra RK, et al. Prevention of cardiac hypertrophy and heart failure by silencing of NF-kappaB. J Mol Biol 2008;375:637-49. [PubMed]


