High mobility group box 1: a novel mediator of Th2-type response-induced airway inflammation of acute allergic asthma
Background
High mobility group box 1 (HMGB1) is a transcription factor present in the nucleus of all mammalian cells. When in the inflammatory situation, HMGB1 is secreted into the extracellular environment by activated macrophages NK cells and dendritic cells (1-3). HMGB1 is also released by cells undergoing necrosis and apoptosis (3,4). Excitingly, HMGB1 has been found in the serum of patients with acute and chronic inflammatory conditions, such as sepsis, acute lung injury and rheumatoid arthritis (5-8), suggesting that extracellular HMGB1 could be regarded as a biomarker of tissue injury and a mediator of inflammatory response.
Recently, some studies have further confirmed that HMGB1 could act as a critical mediator to initiate innate and adaptive immune response in inflammation. The anti-HMGB1 neutralizing antibody ameliorated neutrophilic inflammation in airway by suppressing dendritic cell-mediated Th17 polarization (9). On the other hand, HMGB1 induced IL-17-producing alloreactive T cells to mediate allograft rejection in early stage (10). Moreover, HMGB1 could provoke a dose-dependent and a time-dependent increase in Th2, resulting a decreased ratio of Th1 to Th2 in vitro (11).
It’s well known that allergic asthma is a complex disease characterized by chronic and persistent inflammation, especially the eosinophilic inflammation in the respiratory tract (12). The dominance of Th2-type responses has been considered as the significant pathogenesis of acute allergic asthma, which means Th2-mediated eosinophilic inflammation in airway is the major characteristic of allergic asthma (13). Interestingly, previous clinical studies reported that the levels of HMGB1 in induced sputum and plasma were significantly higher in asthmatic patients than those in the healthy controls (14,15).
However, the relationship between HMGB1 and acute allergic asthma is still not very clear. Thus, we speculated that HMGB1 may play an important role in the pathogenesis of acute allergic asthma based on the present understanding on HMGB1 and this disease. In this study, we investigated the potential role of HMGB1 in the pathogenesis of acute allergic asthma by establishing mice models, and further explored its possible mechanism.
Materials and methods
Animals
Female BALB/c mice (6-8 weeks old, weighing 18±2 g) were provided by Laboratory Animals Center of Guilin Medical University and housed in the SPF animal facility under a 12:12 h light/dark photocycle. They are provided with an OVA free diet and water ad libitum. All experimental procedures were approved by the Animal Care and Use Committee of Guilin Medical University.
Induction of acute allergic asthma in mice
Forty-eight female BABL/c mice were divided into four groups (each group had twelve mice): Control group; Asthma group; asthmatic mice with intraperitoneal (i.p.) injection of HMGB1 (Wellbiology Inc., China) (HMGB1 group); and asthmatic mice with i.p. injection both HMGB1 and anti-HMGB1 (monoclonal antibody of HMGB1, Wellbiology Inc., China). Mice were sensitized by i.p. injection of 0.01 mg OVA, which was (Grade V; Sigma) emulsified in 2 mg of aluminum hydroxide gel, in a total volume of 200 µL on days 1 and 13 as our previous study described (16). Mice were challenged with aerosolized 5% OVA for 30 min between days 19 and 24 (PARI BOY CE, German). Mice in the control group were sensitized and provocated with normal saline instead of OVA. HMGB1 (10 μg/g mouse) was administered by i.p. 30 min before each OVA aerosol challenge, and anti-HMGB1 (10 μg/g mouse) was used by i.p. 30 min before using HMGB1.
Assessment of pulmonary function
Methacholine-induced airway hyperresponsiveness (AHR) was assessed at 24 h after the last OVA or saline inhalation challenge by using an animal airway resistance recorder (Buxco Pulmonary Function Apparatus; Buxco Research Systems, USA). Briefly, after anesthesia with 5% Hydrated chlorine aldehyde, mice were implemented with tracheal intubation and connected with small animal respirator. Then mice were exposed for 3 min to nebulized saline to establish baseline of measured airway resistance and dynamic lung compliance (Cdyn). Subsequently, they were exposed to increasing concentrations of aerosolized methacholine (MCh: 0.78-12.5 mg/mL; Sigma) in saline and ventilated with a small animal respirator (DHX-50, China) delivering 0.01 mL/g bodyweight at a rate of 120 bpm and a rate of 1:1 of I:E. Following each nebulization, records were taken every 5 min. The averages of those records were MCh concentrations, namely 0.78, 1.56, 3.12, 6.25, 12.5 mg/mL.
Cytological analysis and ELISA in bronchoalveolar lavage fluid (BALF)
As described in our previous study (16), bronchoalveolar lavage (BAL) was performed by infusion and extraction of 0.5 mL of ice-cold saline with 1% Bovine Serum Albumin (BSA, Beyotime) after anaesthetization with 5% Hydrated chlorine aldehyde at a dose of 500 mg/kg. Each BAL sample was centrifugated immediately and then the supernatant were collected. The samples were stored under the −70 °C for detecting the levels of IFN-γ, IL-4, IL-5, IL-6, IL-8, IL-17 and HMGB1 in asthma group and control group by ELISA. In addition, total number of viable cells was counted in a hemocytometer using trypan blue exclusion. The eosinophils, neutrophils, lymphocytes and macrophages were stained by hematoxylin and eosin (H-E) and determined on cytospin smears. Their numbers were counted as well.
Histological analysis
The lungs tissues were fixed in 4% paraformaldehyde overnight and embedded in paraffin. Lung sections of 3 μm were stained with H-E or with Periodic acid Schiff (PAS) reagent and examined with a Leica microscope. Peribronchial infiltrates and goblet cells hyperplasia were assessed by a semiquantitative score by two observers independently. And the severity of peribronchial inflammation was graded semiquantitatively using the scale: 0, normal; 1, a few cells; 2, a ring of inflammatory cells one cell layer deep; 3, a ring of inflammatory cells two to four cells deep; 4, a ring of inflammatory cells more than four cells deep. To determine the extent of mucus production, numerical scores for goblet cell hyperplasia in each airway were estimated as following: 0, no goblet cells; 1, <25%; 2, 25-50%; 3, 50-75% (including 50%); and 4, >75%.
Western blot analysis
Nuclear and cytoplasmic protein was extracted from at least three different lung tissue samples from each group. The protein was resolved by 10% SDS-PAGE. Immunoblot analysis was performed by transferring the proteins onto polyvinylidene difluoride membranes (Millipore) using a Mini Trans Blot system (Bio-Rad). The membranes were blocked with 5% milk in Tris Buffered Saline with Tween 20 (TBST) and incubated overnight at 4 °C with antibodies against HMGB1, T-bet or GATA3. It was followed by incubation with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. And the following analyses were carried out using an established technique (16).
Statistical analysis
Significant differences were evaluated by Student’s t-test (two-tailed) or one-way ANOVA test. P values ≤0.05 were considered statistically significant. All the studies were conducted independently for three times and the data were expressed as mean ± standard deviation (SD). The statistical analyses were performed using SPSS 18.0.
Results
Up-regulation of HMGB1 expression in the lung tissue and changes of HMGB1 production in the BALF and lung homogenate
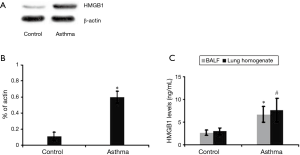
To better explore the role of HMGB1 in the acute allergic asthma, we detected the expression of HMGB1 protein in the lung tissue of mice (Figure 1A). We found that HMGB1 expression in the lung tissue of asthmatic mice was significantly increased compared to control group (P<0.01; Figure 1B). We also measured the levels of HMGB1 in the BALF, lung homogenate and plasma from mice. Our study showed that HMGB1 levels in both BALF and lung homogenate of asthma mice were all much higher than those in control group, which were similar to the previous study on the asthmatic patients (P<0.01; Figure 1C) .
Neutralization of HMGB1 signalling could improve the pulmonary function of acute allergic asthma
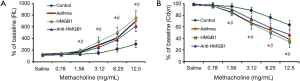
As we know, allergic asthma is a persistent inflammatory disorder in the lung with a prevailed Th2 immune response to inhaled allergens, which leads to AHR. Thus, AHR is one of the characteristics to identify acute allergic asthma. In our study, AHR is assessed by pulmonary function test including measuring airway resistance of lung (RL) and Cdyn via MCh challenge. The allergic phenotype in female BALB/c mice was induced essentially as described for OVA-induced asthma. Compared to saline-challenged group, OVA-challenged group mice were showed distinct AHR, which was typically manifested by a high RL (211.79 vs. 410.15 when in a concentration of 6.25 mg/mL; P<0.01, Figure 2) and a low Cdyn (76.09 vs. 54.05 when in a concentration of 6.25 mg/mL; P<0.01, Figure 2) in response to MCh. However, when exogenous HMGB1 was i.p. injected into the mice, AHR of the asthma mice was more serious, and the RL become higher and Cdyn become lower than those without HMGB1 injection. Whereas neutralizing monoclonal antibody of HMGB1 was administrated at 30 min before exogenous HMGB1, the above effect of HMGB1 on AHR was fully abrogated as the Figure 2 showed (P<0.01), which suggested that neutralization of HMGB1 signaling could strikingly improve pulmonary function of mice model with acute allergic asthma.
Effects of HMGB1 on the levels of related inflammatory cytokines in the BALF and lung homogenate
Both Th1-derived cytokine IFN-γ and Th2-derived cytokines IL-4, IL-5 play an important role in the promotion of eosinophilic inflammation in the airway of allergic asthma. IL-6 and IL-8 were also observed because HMGB1 signaling is related with neutrophilic inflammation. In our study, the above cytokines in BALF and lung homogenate from each group were detected by ELISA. It was noted that the levels of IFN-γ ((31.43±12.75 vs. 66.56±11.45 pg/mL in BALF and 147.74±31.59 vs. 208.53±20.74 pg/mL in lung homogenate. P<0.01 respectively; Figure 3A) in asthma group were lower than those in control group, whereas the levels of IL-4 (79.43±29.75 vs. 19.75±9.44 pg/mL in BALF and 159.69±46.67 vs. 50.54±14.62 pg/mL in lung homogenate. All P values were<0.01; Figure 3B) and IL-5 (Figure 3C) were significantly higher. Particularly, exogenous HMGB1 significantly increased the secretion of Th2-related cytokines, which could be reversed by HMGB1 neutralization as the Figure 3 showed. In addition, exogenous HMGB1 could induce an abundance of IL-6 and IL-8 in the lung (P<0.01 respectively; Figure 3D,E), suggesting an aggravated neutrophilic inflammation in the airway.

Moreover, we detected the levels of cytokine IL-17 in the BALF and lung homogenate, which is considered to be the cytokine responded to Th17 activity and be related with neutrophils recruitment and activation. In our study, the level of IL-17 in the BLAF and lung homogenate were much higher than those in the control group after HMGB1 administration, which could be abrogated by anti-HMGB1 (P<0.01; Figure 3F).
HMGB1 could promote recruitment of OVA-induced inflammatory cells in the BALF
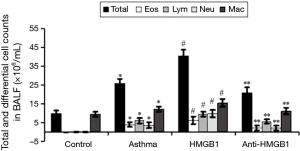
We investigated the regulatory role of HMGB1 in OVA-induced inflammatory cells recruitment. Inflammatory cells recruiting into the lung tissue and the alveolar space were analyzed through BAL, and then BALF was collected 24 h after the last aerosol challenge. The total number of cells in BALF from OVA-challenged mice was about three times as those from saline-treated controls (9.79×106 to 25.64×106 cells/mL, Figure 4). The increased number of cells was mainly due to eosinophil and neutrophil recruitment. HMGB1 injection could significantly increase the total number of cell in BALF by 59% (25.64×106 to 40.65×106 cells/mL, P<0.01), the number of eosinophil by 61% (3.86×106 to 6.2×106 cells/mL, P<0.01), neutrophil by 168% (3.63×106 to 9.73×106 cells/mL, P<0.01), lymphocyte by 55% (6.05×106 to 9.36×106 cells/mL, P<0.01) and macrophage by 27% (12.1×106 to 15.36×106 cells/mL, P<0.01), compared with those in asthma group without HMGB1 injection. However, those effects could be abrogated by neutralizing anti-HMGB1 monoclonal antibody. As the figure described, HMGB1 inhibition could significantly reduce the numbers of eosinophil, lymphocyte and neutrophil (P<0.01, respectively, Figure 4), which suggested that HMGB1 could promote the recruitment of inflammatory cells in the airway of acute allergic asthma.
Exogenous HMGB1 enhances airway inflammation and mucus production of acute allergic asthma
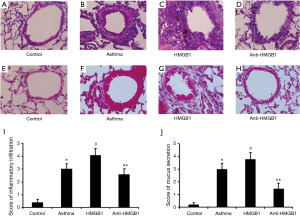
We further investigated the effects of HMGB1 on the inflammation and mucus production in the airway. Histological analysis showed peribronchial cell recruitment and hyperplasia of goblet cell in the lung tissue of OVA-challenged mice.It was noted that control group was showed normal (Figure 5A), whereas asthma group was showed a distinct infiltration of inflammatory cells into the peribronchiolar and perivascular connective tissues (Figure 5B). Furthermore, exogenous HMGB1 could promote inflammatory infiltration (Figure 5C), which could be attenuated by neutralizing anti-HMGB1 antibody (Figure 5D). Compared with the control mice (Figure 5E), marked goblet cell hyperplasia and mucus hypersecretion within the bronchi were observed in asthma mice (Figure 5F). Similarly, as the figure showed, goblet cell hyperplasia and mucus secretion were promoted by HMGB1 administration (Figure 5G), whereas anti-HMGB1 antibody could reverse this effect (Figure 5H). Finally, these results were confirmed by semiquantitative analysis (Figure 5I,J), which suggested that exogenous HMGB1 could aggravate inflammation of airway in the acute allergic asthma.
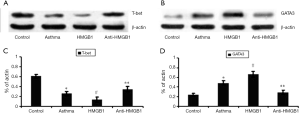
HMGB1 promotes the expression of GATA3 protein while reduces T-bet protein expression in acute allergic asthma
HMGB1 could regulate the levels of Th1/Th2-derived cytokines such as IFN-γ, IL-4, IL-5 in the airway of acute allergic asthma. In order to observe the effects of HMGB1 on Th1/Th2 profile, we detected the expression of T-bet and GATA3 proteins based on the fact that CD4+ T cell polarization is governed by lineage-specific transcription factors and T-bet, GATA3 is the Th1 and Th2 cell lineage transcription factor respectively. Then T-bet protein and GATA3 protein were extracted from lung tissue of each group, and were measured by Western blot. Our results showed that exogenous HMGB1 could reduce the expression of T-bet protein (Figure 6A) in the lung tissue but promote the expression of GATA3 protein (Figure 6B) and the statistical graph was also showed (T-bet, Figure 6C and GATA3, Figure 6D). In contrast, blockade of HMGB1 with monoclonal antibody significantly reversed those effects in above, which indicates that HMGB1 signaling played a role in the pathogenesis of acute allergic asthma through regulating the Th2-type response.
Discussion
In this study, we found that HMGB1 signaling was involved in the pathogenesis of acute allergic asthma. Our results first suggested that exogenous HMGB1 could aggravate the eosinophilic airway inflammation of acute allergic asthma, in which a dominance of Th2-type response induced by HMGB1 may play an important role. In addition, the recruitment and infiltration of neutrophils in the airway was also promoted by HMGB1 administration in this study.
HMGB1 is a highly conserved and ubiquitous protein, which is identified and purified from nuclei with histones (17). Generally, HMGB1 is expressed in the nucleus of a wide variety of cells. In inflammatory status, HMGB1 is produced and released into extracellular milieu and then be involved into inflammatory responses. As mentioned before, HMGB1 can be actively released from inflammatory cells including macrophages, monocytes, dendritic cells, endothelial cells, whereas it is passively released from necrotic or damaged cells (2-4). Actually, as an inflammatory mediator, previous studies have pointed out that HMGB1 plays an important role as mediator in many inflammatory diseases such as acute lung injury, arthritis, cancers or sepsis through promoting local and systemic inflammation (3,18).
Recently, several studies found that HMGB1 levels in induced sputum and plasma from asthmatic patients were significantly higher than those in control subjects, which were correlated with the severity of asthma (14,15,19). In cystic fibrosis airway disease, HMGB1 contributes to the pulmonary inflammation and lung matrix degradation (20,21). In our study, we confirmed that the level of HMGB1 in acute allergic asthma was remarkably higher than that in the control group based on the animal experiment model. These findings suggest that HMGB1 may play a role in the pathogenesis of allergic asthma.
It has been found that HMGB1 is positively correlated with the number of neutrophils (5,22). The neutrophilic inflammation in the airways contributes to the severity of asthma (23-25). In our study, we found that exogenous HMGB1 injection could lead to a significantly increased number of neutrophils in the BALF and aggravate asthma symptom of experimental animals compared with controls, which further supports that the production of HMGB1 is related with the severity of asthma (19). Moreover, in order to investigate the possible pathway to regulate neutrophilic inflammation by HMGB1 in asthma, we detected the expression of cytokines IL-6 and IL-8, which are major mediators and promoters for recruitment and activation of neutrophils (26,27). Our results showed that the levels of IL-6 and IL-8 in the lung were much higher than those in control group after exogenous HMGB1 injection, which could be abrogated by anti-HMGB1 neutralizing antibody. Therefore, our results implied that HMGB1 could promote neutrophilic inflammation in the airway of acute allergic asthma through regulating IL-6 and IL-8.
In addition, IL-17 level in the airway was promoted by exogenous HMGB1 in present study. IL-17 is generally produced by Th17 cells, which belong to the CD4+ T lymphocyte family (28). And IL-17 has been deemed to the characteristic cytokine for Th17 cells in the CD4+ T subsets (29). Recent study demonstrated that exogenous IL-17 protein could recruit neutrophils partly via inducing a release of IL-8 and IL-6 in rat airways in vivo (30-32). Although Th2 and Th17 cells are able to induce AHR, only Th17 cell-mediated airway inflammation and AHR are steroid-resistant (33). Thus, we suggested that Th17/IL-17 pathway may play a key role when HMGB1 promotes inflammation in asthma. This deserves further research in future, especially its potential role in steroid-resistant asthma.
Allergic asthma is a complicated inflammatory disease in airway, in which eosinophilic inflammation has been widely accepted as its characteristic, although neutrophils infiltration in the airway also plays a role (34,35). However, the role of HMGB1 in the eosinophilic inflammation of acute allergic asthma is still uncertain. In this study, one of the major objectives is to investigate the effect of HMGB1 on the eosinophilic inflammation in the airway of acute allergic asthma through establishing OVA-induced mice models. Our results first indicated that exogenous HMGB1 could promote the eosinophilic inflammation in the airway and aggravate the airflow limitation based on pulmonary function test in vivo, which confirmed that HMGB1 is indeed involved in the pathogenesis of acute allergic asthma as we mentioned before. The possible mechanism of HMGB1 promoting eosinophilic inflammation needs our further investigation.
In recent years, several studies suggested that HMGB1 participated in the regulation of immune response. The dysfunction of immune regulation in the airway has been thought to be an important mechanism for acute allergic asthma. Shi et al. found that enhanced HMGB1 expression may contribute to Th17 cells activation in rheumatoid arthritis, which could be confirmed by exogenous HMGB1 administration in vitro (36). More interestingly, HMGB1 could provoke a dose-dependent and time-dependent increase of Th2 number and reduce the ratio of Th1/Th2 in vitro (11). So we speculated that HMGB1 may promote Th2-type response in airway, which could induce the eosinophilic inflammation of acute allergic asthma (37,38). Therefore, we detected the effect of HMGB1 on the expression of Th2 cells transcriptional factor GATA3 and cytokine IL-4 in the lung of asthma mice (39-41). Our results showed that HMGB1 significantly enhanced the expression of GATA3 and secretion of IL-4, IL-5, but suppressed the expression of T-bet and the secretion of IFN-γ in the lung (41,42). Considering the important role of IL-4 and IL-5 in the promotion of allergic eosinophilic inflammation in the airway (34), we suggested that HMGB1 may aggravate the eosinophilic inflammation of acute allergic asthma by inducing a dominance of Th2-type response.
Conclusions
In conclusion, our study first found that HMGB1 could aggravate the airway inflammation of acute allergic asthma by promoting a dominance of Th2-type response. HMGB1 could promote both the eosinophilic inflammation and neutrophilic inflammation in the airway. These findings revealed a great potential of HMGB1 signaling as a biomarker and therapeutic target for asthma.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81560007 and No. 81360006) Natural Science Foundation of Guangxi (No. 2015GXNSFAA139106 and No. 2013GXNSFBA019175).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999;285:248-51. [PubMed]
- Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 2000;192:565-70. [PubMed]
- Yang H, Tracey KJ. Targeting HMGB1 in inflammation. Biochim Biophys Acta 2010;1799:149-56.
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002;418:191-5. [PubMed]
- Wang H, Ward MF, Sama AE. Novel HMGB1-inhibiting therapeutic agents for experimental sepsis. Shock 2009;32:348-57. [PubMed]
- Ueno H, Matsuda T, Hashimoto S, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med 2004;170:1310-6. [PubMed]
- Ogawa EN, Ishizaka A, Tasaka S, et al. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am J Respir Crit Care Med 2006;174:400-7. [PubMed]
- Hamada T, Torikai M, Kuwazuru A, et al. Extracellular high mobility group box chromosomal protein 1 is a coupling factor for hypoxia and inflammation in arthritis. Arthritis Rheum 2008;58:2675-85. [PubMed]
- Zhang F, Huang G, Hu B, et al. Anti-HMGB1 neutralizing antibody ameliorates neutrophilic airway inflammation by suppressing dendritic cell-mediated Th17 polarization. Mediators Inflamm 2014;2014:257930.
- Duan L, Wang CY, Chen J, et al. High-mobility group box 1 promotes early acute allograft rejection by enhancing IL-6-dependent Th17 alloreactive response. Lab Invest 2011;91:43-53. [PubMed]
- Huang LF, Yao YM, Meng HD, et al. The effect of high mobility group box-1 protein on immune function of human T lymphocytes in vitro. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2008;20:7-13. [PubMed]
- Ngoc PL, Gold DR, Tzianabos AO, et al. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol 2005;5:161-6. [PubMed]
- Umetsu DT, McIntire JJ, Akbari O, et al. Asthma: an epidemic of dysregulated immunity. Nat Immunol 2002;3:715-20. [PubMed]
- Hou C, Zhao H, Liu L, et al. High mobility group protein B1 (HMGB1) in Asthma: comparison of patients with chronic obstructive pulmonary disease and healthy controls. Mol Med 2011;17:807-15. [PubMed]
- Watanabe T, Asai K, Fujimoto H, et al. Increased levels of HMGB-1 and endogenous secretory RAGE in induced sputum from asthmatic patients. Respir Med 2011;105:519-25. [PubMed]
- Ma L, Zeng J, Mo B, et al. ANP/NPRA signaling preferentially mediates Th2 responses in favor of pathological processes during the course of acute allergic asthma. Int J Clin Exp Med 2015;8:5121-8. [PubMed]
- Wen L, Huang JK, Johnson BH, et al. A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1. Nucleic Acids Res 1989;17:1197-214. [PubMed]
- Mitola S, Belleri M, Urbinati C, et al. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol 2006;176:12-5. [PubMed]
- Zhou Y, Jiang YQ, Wang WX, et al. HMGB1 and RAGE levels in induced sputum correlate with asthma severity and neutrophil percentage. Hum Immunol 2012;73:1171-4. [PubMed]
- Gaggar A, Rowe SM, Matthew H, et al. Proline-Glycine-Proline (PGP) and High Mobility Group Box Protein-1 (HMGB1): Potential Mediators of Cystic Fibrosis Airway Inflammation. Open Respir Med J 2010;4:32-8. [PubMed]
- Rowe SM, Jackson PL, Liu G, et al. Potential role of high-mobility group box 1 in cystic fibrosis airway disease. Am J Respir Crit Care Med 2008;178:822-31. [PubMed]
- Ding HS, Yang J, Gong FL, et al. High mobility group [corrected] box 1 mediates neutrophil recruitment in myocardial ischemia-reperfusion injury through toll like receptor 4-related pathway. Gene 2012;509:149-53. [PubMed]
- Green RH, Brightling CE, Woltmann G, et al. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 2002;57:875-9. [PubMed]
- Berry M, Morgan A, Shaw DE, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax 2007;62:1043-9. [PubMed]
- Shaw DE, Berry MA, Hargadon B, et al. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest 2007;132:1871-5. [PubMed]
- Taylor PR, Roy S, Leal SM Jr, et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nat Immunol 2014;15:143-51. [PubMed]
- Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest 2001;119:1329-36. [PubMed]
- Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005;6:1123-32. [PubMed]
- Fouser LA, Wright JF, Dunussi-Joannopoulos K, et al. Th17 cytokines and their emerging roles in inflammation and autoimmunity. Immunol Rev 2008;226:87-102. [PubMed]
- Essilfie AT, Simpson JL, Horvat JC, et al. Haemophilus influenzae infection drives IL-17-mediated neutrophilic allergic airways disease. PLoS Pathog 2011;7:e1002244. [PubMed]
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008;28:454-67. [PubMed]
- Gaffen SL. An overview of IL-17 function and signaling. Cytokine 2008;43:402-7. [PubMed]
- McKinley L, Alcorn JF, Peterson A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol 2008;181:4089-97. [PubMed]
- Ngoc PL, Gold DR, Tzianabos AO, et al. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol 2005;5:161-6. [PubMed]
- Macdowell AL, Peters SP. Neutrophils in asthma. Curr Allergy Asthma Rep 2007;7:464-8. [PubMed]
- Shi Y, Sandoghchian Shotorbani S, Su Z, et al. Enhanced HMGB1 expression may contribute to Th17 cells activation in rheumatoid arthritis. Clin Dev Immunol 2012;2012:295081.
- Umetsu DT, McIntire JJ, Akbari O, et al. Asthma: an epidemic of dysregulated immunity. Nat Immunol 2002;3:715-20. [PubMed]
- Umetsu DT, DeKruyff RH. The regulation of allergy and asthma. Immunol Rev 2006;212:238-55. [PubMed]
- Farrar JD, Ouyang W, Löhning M, et al. An instructive component in T helper cell type 2 (Th2) development mediated by GATA-3. J Exp Med 2001;193:643-50. [PubMed]
- Zhou M, Ouyang W. The function role of GATA-3 in Th1 and Th2 differentiation. Immunol Res 2003;28:25-37. [PubMed]
- Grogan JL, Mohrs M, Harmon B, et al. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity 2001;14:205-15. [PubMed]
- Szabo SJ, Kim ST, Costa GL, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000;100:655-69. [PubMed]


