Pharmacological treatment response according to the severity of symptoms in patients with chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is usually progressive. Spirometry is the gold-standard measurement of airflow limitation, and post-bronchodilator forced expiratory volume in 1 second (FEV1) has been used for the Global Obstructive Lung Disease (GOLD) spirometric grading system (1). Pharmacotherapy for patients with COPD is used to reduce symptoms and exacerbations and to improve health status and exercise tolerance. Until 2011, GOLD guidelines had recommended pharmacological treatment based solely on the post-bronchodilator FEV1, and patient symptoms and health status assessments did not guide the choice of pharmacological treatment. However, FEV1 not only correlates poorly with the severity of breathlessness, exercise intolerance, and health status (2,3) but the measure also is not necessarily indicative of treatment efficacy (4,5). The 2011 revised GOLD guidelines changed the pharmacological treatment paradigm based on the combined assessment of symptoms and risk of exacerbations with the patient’s spirometric severity classification (1,6). The Modified British Medical Research Council (mMRC) dyspnea scale and the COPD Assessment Test (CAT) are recommended measures for assessing symptoms. An mMRC ≥2 and a CAT ≥10 are used as a cutoff for a high-level symptoms. Regular treatment with long-acting bronchodilators is recommended for patients with high-level symptoms, and inhaled corticosteroids (ICS) are recommended for patients with severe or very severe COPD with frequent exacerbations. However, the evidence for pharmacotherapy for COPD is mostly based on the severity of airflow limitation (FEV1% of predicted), and no clinical trials have reported outcomes of pharmacotherapy based on the severity of symptoms.
We hypothesized that the mMRC dyspnea scale and the CAT score would be related to the pharmacological treatment effects in patients with COPD. The aim of this study was to determine the relationship between baseline mMRC dyspnea scale and the CAT score and their changes after a 3-month treatment with an inhaled long-acting muscarinic antagonist (LAMA) and/or ICS/long-acting beta2-agonist (LABA) combinations.
Materials and methods
Patients
A total of 102 stable COPD patients selected from the Korean Obstructive Lung Disease (KOLD) cohort were included in this study. All had stable COPD and were prospectively recruited from the pulmonary clinics of six tertiary hospitals in South Korea between June 2012 and August 2014. The study design and outcomes of the KOLD cohort have been described elsewhere (7). COPD was diagnosed based on the presence of airflow limitation that was not fully reversible [post-bronchodilator FEV1/forced vital capacity (FVC) <70%] and smoking history (>10 pack-years). Key exclusion criteria were COPD exacerbations or lower respiratory infections within 8 weeks before the screening visit, diagnosis of asthma and/or other relevant lung disease except COPD, and requiring supplemental use of oxygen. This study was approved by the Asan Medical Center Institutional Review Boards (IRBs) (the main study site), and each patient provided written informed consent.
Study design
Baseline clinical data were obtained after discontinuation of the ICS/LABA combinations for 1 week, discontinuation of LAMA for 2 days, or discontinuation of an inhaled short-acting beta2-agonist for 12 h. The baseline clinical data included demographic information, smoking history, medical history, medications, pulmonary function tests, and a six-minute walk test. The mMRC dyspnea scale and the validated Korean version of the CAT and the St. George’s Respiratory Questionnaire (SGRQ) questionnaire were administered to assess the degree of dyspnea and health-related quality of life (HRQoL) (8,9). After the enrollment visit, all patients were recommended for treatment with an inhaled LAMA and/or ICS/LABA combinations. During the treatment period, only salbutamol was allowed as needed. After a 3-month treatment, the mMRC dyspnea scale, the CAT questionnaire, and spirometry were performed 2 to 6 h after inhalation of the drugs in the morning. Adherence to the treatment medication was monitored and recorded by research coordinators. All included patients were asked to bring back their inhalation device in order to measure how much they had used. All patients included in the study indicated that they had taken >80% of the prescribed respiratory medication doses. All pulmonary function tests were carried out as recommended by the American Thoracic Society/European Respiratory Society (10-12).
Statistical analysis
Data are summarized as the means and standard errors for subgroup information for continuous variables and as relative frequencies for categorical variables. The groups were compared using a Pearson’s χ2 test, Fisher’s exact test, Student’s t-test, or Mann-Whitney U test depending on the type of variable and its distribution. The relationship between two continuous variables was measured by a Pearson or Spearman correlation analysis. Pharmacological treatment responses were assessed by changes in the mMRC dyspnea scale, the CAT scores, FEV1, and FVC before and after the 3-month treatment. The numbers of patients were calculated that changed more than the minimal clinically important difference (MCID) for the mMRC dyspnea scale and the CAT score after a 3-month treatment. The MCIDs for the mMRC dyspnea scale and the CAT score were 1 and 2 points, respectively (13,14). To determine the predictors of symptom improvement after the 3-month treatment, a multiple analysis was performed using multiple logistic regression models for the mMRC dyspnea scale and the CAT score changes greater than their MCIDs adjusting for age, body mass index, smoking pack-years, current smoking, FEV1 (% predicted), diffusing capacity for carbon monoxide (% predicted), and inspiratory capacity (IC)/total lung capacity (TLC). Multiple linear regression analysis was also performed to identify the clinical factors associated with spirometric pulmonary function improvement after a 3-month treatment. All statistical analyses were carried out using the IBM SPSS statistical package (version 21.0; IBM Corp., Armonk, NY, USA). The significance threshold was defined as a P<0.05.
Results
Baseline characteristics of the enrolled patients
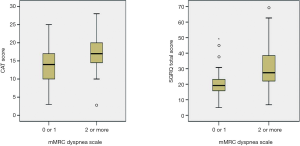
All the enrolled patients were male, had a mean age of 67.1 years [standard deviation (SD) 6.7] years, and had a mean post-bronchodilator FEV1 of 52.9% (SD: 14.1) of the predicted value. The GOLD spirometric severity grade of the included patients was distributed as follows: 2 (2%) GOLD I (mild), 64 (63%) GOLD II (moderate), 29 (28%) GOLD III (severe COPD), and 4 (7%) GOLD IV (very severe). The mean baseline mMRC dyspnea scale was 1.4 (0.8), the CAT score was 15.0 (5.5), and the SGRQ score was 24.2 (12.0). The mMRC dyspnea scales were significantly correlated with the CAT scores and the SGRQ scores (Spearman’s rho =0.288, P=0.003 and 0.450, P≤0.001). The CAT scores were also significantly correlated with the SGRQ scores (Pearson’s r =0.586, P<0.001). Sixty-two (61%) patients with an mMRC dyspnea scale 0 or 1 were classified to the “less dyspnea” group (9 mMRC 0 and 53 mMRC 1), and 40 (39%) patients with a mMRC dyspnea scale ≥2 to the “more dyspnea” group (30 mMRC 2, 9 mMRC 3, and 1 mMRC 4). Baseline characteristics according to the mMRC dyspnea scale group are summarized in Table 1. The “more dyspnea” group had a significantly lower FEV1, FVC, and DLco and higher RV % predicted values than the “less dyspnea” group. The “more dyspnea” group had significantly higher CAT and SGRQ scores than the “less dyspnea group”, and the differences between two groups were greater than their MCIDs (CAT ≥−2 and SGRQ ≥−4). However, there was considerable overlap in the CAT and SGRQ score distributions between these two groups (Figure 1). In the “less dyspnea” group 77% of patients had a CAT score ≥10, and 19% of patients had a SGRQ score ≥25.
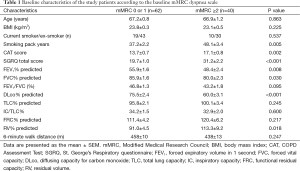
Full table
Symptom and pulmonary function changes after a 3-month treatment
During the 3-month treatment period, 12% of the enrolled patients were treated with an inhaled LAMA (tiotropium 18 µg once daily), 9% with ICS/LABA combinations (salmeterol/fluticasone 50/250 µg or formoterol/budesonide 9/320 µg twice daily), and 78% with inhaled LAMA and ICS/LABA combinations. There were no significant differences in the 3-month treatment regimens between the “less dyspnea” and the “more dyspnea” groups. Table 2 summarizes the symptom scores and spirometric pulmonary function changes after a 3-month treatment according to the baseline mMRC dyspnea scale group. After the 3-month treatment, the mean mMRC dyspnea scale in the “more dyspnea” group was more significantly decreased than that in the “less dyspnea” group. The proportion of patients showing more mMRC improvements than their MCIDs was also much larger in the “more dyspnea” group than that in the “less dyspnea” group (50% vs. 4.8%, P<0.001). However, there were no significant differences in the CAT score or spirometric pulmonary function changes between the two groups. Baseline mMRC scales (Spearman’s rho =−0.591, P<0.001) and baseline CAT scores (Pearson’s r =−0.337, P=0.001) were significantly correlated with their changes after a 3-month treatment (Figure 2). Multiple logistic regression analysis demonstrated that baseline mMRC scales and CAT scores were the only independent predictors of their respective improvement greater than MCIDs after the 3-month treatment (Table 3). Multiple linear regression analysis demonstrated that spirometric pulmonary function changes after the 3-month treatment had no association with the baseline mMRC dyspnea scale or CAT score. The baseline FEV1% predicted and FVC% predicted were only associated with their respective changes after a 3-month treatment (Table 4).

Full table

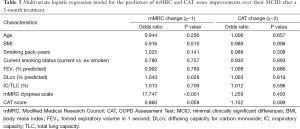
Full table

Full table
Discussion
To our knowledge, our present study is the first to analyze the pharmacological treatment response based on stratifying the symptom severity levels. The current evidence for pharmacological treatment of COPD is primarily based on the severity of airflow limitation; however, no studies to date have reported on the pharmacological treatment effects based on changes in the severity of symptoms. Our current study results demonstrate several clinically important findings about the response to pharmacotherapy in patients with COPD. First, the baseline severity of COPD symptoms was associated with patient response to pharmacotherapy. COPD patients with a higher baseline mMRC dyspnea scale and CAT score experienced greater symptom reduction from pharmacotherapy. Second, the baseline mMRC dyspnea scale and the CAT score can predict the response to pharmacotherapy. Third, even patients with a mMRC dyspnea scale ≤1 may receive significant symptom relief that can be measured by the CAT score. Finally, the baseline FEV1 cannot predict the symptomatic response to pharmacotherapy.
Since no existing medications have been shown to modify the long-term decline in lung function in COPD, the current major goals for pharmacological treatment of this disease are to reduce current symptoms and future exacerbation risks and to improve the patient’s health status (1). Thus, identifying the clinical parameters that can be used to predict symptom relief and health status improvement after pharmacological treatment is clinically important. COPD is a multi-component and heterogeneous disease with variable clinical presentation and treatment responses (15,16). FEV1 has been recognized as the gold standard index of airflow limitation that measures both symptomatic relief and disease progression (17). However, FEV1 demonstrates only a weak correlation with patient-centered outcomes, such as dyspnea, exercise capacity, and HRQoL (2,3). Furthermore, FEV1 has limited usefulness in the evaluation of therapeutic response in individual patients (16,18). Thus, a comprehensive assessment of pulmonary function, symptoms, and HRQoL is required to adequately evaluate the expected therapeutic response in patients with COPD.
In our current analyses, the mMRC dyspnea scale and the CAT score were used for the quantitative assessment of dyspnea and HRQoL in patients with COPD. Dyspnea is the most bothersome symptom and a major cause of disability in patients with COPD (19). The mMRC dyspnea scale is a simple and reliable measurement of dyspnea associated with daily activity. The scale relates well to other measurements of health status and predicts the risk of future mortality (20). The disadvantage of the mMRC dyspnea scale is its insensitivity to change in response to treatment, and the scale does not take into account the variation in effort exerted by patients in their activities (21). However, a difference of one grade is considered indicative of a clinically significant change in dyspnea (13). The CAT score was developed to measure the impact of COPD on HRQoL and to aid patient-physician communication (22). The CAT score also correlates closely with health status measured using the SGRQ and is reliable and responsive to treatment (23,24). A CAT score ≥10 has been shown to have a significant impact on the daily lives of patients with COPD and to predict future exacerbations (25,26). An MCID for the CAT has not been officially established, but the threshold has been estimated to be two units (14).
Our present study results support the 2011 revised GOLD pharmacological treatment paradigm based on stratified symptom levels. Regular treatment with long-acting bronchodilators is recommended for COPD patients in the high-symptom group. Current GOLD guidelines recommend a mMRC dyspnea scale ≥2 and a CAT score ≥10 as equivalent symptom cutoffs for categorizing patients into low- or high-symptom groups (6). However, recent studies have suggested that a change in the cutoff for the mMRC dyspnea scale from 2 to 1 was needed, because mMRC dyspnea scale 1 was approximately equivalent with CAT score of 10 (27,28). In our current study, the mean CAT scores of patients with mMRC dyspnea scales 1 and 2 were 13 and 17, respectively. Furthermore, after the 3-month treatment 25 of 53 COPD patients with a mMRC dyspnea scale 1 displayed a significant reduction in their CAT score greater than −2. These data may also provide evidence for the future modification of the mMRC dyspnea scale cutoff points in the GOLD 2011 assessment framework.
There were several limitations in this study. First, this study was an open-label observational design that did not include a placebo arm. The likelihood of bias in an open-label study should be considered. Second, approximately 90% of the included patients had moderate to severe airflow limitation (GOLD spirometric grade II or III). Therefore, the potential for selection bias cannot be excluded, and our study results may need further validation in patients with very severe COPD. Third, approximately 80% of the enrolled patients were treated with a triple combination of inhaled LAMA and ICS/LABA in order to maximize the pharmacological treatment effect. In real-world clinical practice, this triple combination therapy is usually used in patients with very severe COPD patients with frequent exacerbations. Further studies may be required to evaluate the effects of mono-therapy with LAMA or LABA based on symptom severity. Finally, all of our enrolled patients were men. Gender may have a substantial influence on the treatment response in COPD patients, so we cannot generalize our present results to women (29).
In conclusion, the baseline severity of COPD symptoms is associated with patient response to pharmacotherapy. COPD patients with a higher baseline mMRC dyspnea scale and CAT score experience a greater symptom reduction from pharmacotherapy.
Acknowledgements
Funding: This work was supported by grants from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare Affairs, Republic of Korea (HI10C2020 and A102065).
Footnote
Conflicts of Interest: JB Seo has been an investigator in a government-sponsored study (2006-2015 Korea Science and Engineering Foundation). YM Oh has been an investigator in university-sponsored studies (Asan Institute for Life Science, University of Ulsan College of Medicine) and an industry-sponsored study (MSD Korea and AstraZeneca Korea), and has participated as a speaker at scientific meetings organized and financed by various pharmaceutical companies (Handok, GlaxoSmithKline, AstraZeneca Korea, MSD Korea and Boehringer Ingelheim) and a publishing company (Korea Doctors’ Weekly). SD Lee serves as a consultant to GlaxoSmithKline and has participated as a speaker at scientific meetings organized and financed by various pharmaceutical companies (GlaxoSmithKline, AstraZeneca Korea and Boehringer Ingelheim). The remaining authors have no conflicts of interest to disclose.
References
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [PubMed]
- Mahler DA, Harver A. A factor analysis of dyspnea ratings, respiratory muscle strength, and lung function in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1992;145:467-70. [PubMed]
- Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax 2001;56:880-7. [PubMed]
- Calverley P, Pauwels RA, Jones PW, et al. The severity of airways obstruction as a determinant of treatment response in COPD. Int J Chron Obstruct Pulmon Dis 2006;1:209-18. [PubMed]
- Jenkins CR, Jones PW, Calverley PM, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res 2009;10:59. [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of COPD. Available online: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed 2015 January.
- Park TS, Lee JS, Seo JB, et al. Study Design and Outcomes of Korean Obstructive Lung Disease (KOLD) Cohort Study. Study Design and Outcomes of Korean Obstructive Lung Disease (KOLD) Cohort Study. Tuberc Respir Dis (Seoul) 2014;76:169-74. [PubMed]
- Hwang YI, Jung KS, Lim SY, et al. A Validation Study for the Korean Version of Chronic Obstructive Pulmonary Disease Assessment Test (CAT). Tuberc Respir Dis (Seoul) 2013;74:256-63. [PubMed]
- Kim YS, Byun MK, Jung WY, et al. Validation of the Korean version of the St. George’s respiratory questionnaire for patients with hronic respiratory disease. Tuberc Respir Dis 2006;61:121-8.
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [PubMed]
- Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26:511-22. [PubMed]
- Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720-35. [PubMed]
- de Torres JP, Pinto-Plata V, Ingenito E, et al. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest 2002;121:1092-8. [PubMed]
- Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med 2014;2:195-203. [PubMed]
- Friedlander AL, Lynch D, Dyar LA, et al. Phenotypes of chronic obstructive pulmonary disease. COPD 2007;4:355-84. [PubMed]
- Lee JS, Huh JW, Chae EJ, et al. Different therapeutic responses in chronic obstructive pulmonary disease subgroups. Int J Tuberc Lung Dis 2011;15:1104-10. [PubMed]
- Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008;31:416-69. [PubMed]
- Newton MF, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest 2002;121:1042-50. [PubMed]
- Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581-6. [PubMed]
- Nishimura K, Izumi T, Tsukino M, et al. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002;121:1434-40. [PubMed]
- Ries AL. Impact of chronic obstructive pulmonary disease on quality of life: the role of dyspnea. Am J Med 2006;119:12-20. [PubMed]
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009;34:648-54. [PubMed]
- Jones PW, Brusselle G, Dal Negro RW, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J 2011;38:29-35. [PubMed]
- Jones PW, Harding G, Wiklund I, et al. Tests of the responsiveness of the COPD assessment test following acute exacerbation and pulmonary rehabilitation. Chest 2012;142:134-40. [PubMed]
- Ringbaek T, Martinez G, Lange P. A comparison of the assessment of quality of life with CAT, CCQ, and SGRQ in COPD patients participating in pulmonary rehabilitation. COPD 2012;9:12-5. [PubMed]
- Lee SD, Huang MS, Kang J, et al. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. Respir Med 2014;108:600-8. [PubMed]
- Jones PW, Adamek L, Nadeau G, et al. Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification. Eur Respir J 2013;42:647-54. [PubMed]
- Kim S, Oh J, Kim YI, et al. Differences in classification of COPD group using COPD assessment test (CAT) or modified Medical Research Council (mMRC) dyspnea scores: a cross-sectional analyses. BMC Pulm Med 2013;13:35. [PubMed]
- Han MK, Postma D, Mannino DM, et al. Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med 2007;176:1179-84. [PubMed]


