Pre-analytic variability in cardiovascular biomarker testing
Introduction
The impact of laboratory medicine on clinical cardiology has dramatically increased over the years (1,2) and a lot of cardiovascular biomarkers have been recently proposed. However, the most widespread biomarkers in cardiology are cardiac troponins and natriuretic peptides, on which this review will focus.
Cardiac troponin I (cTnI) and T (cTnT) have become the most reliable tool for diagnosing acute myocardial infarction (3). According to recent evidence, serial testing with assessment of troponins’ kinetic are recommended if acute cardiac problems are suspected. With the introduction of high sensitivity cardiac troponin assays, quantification of cTn levels is nowadays possible in almost everyone and cTn’s elevation is linked to adverse events (4). cTn rises mainly in acute coronary syndromes but also in chronic or acute renal impairment, hearth failure, sepsis, hypertensive crisis, arrhythmias, pulmonary embolism, myocarditis, stroke and other cardiac problems (5,6).
Nowadays laboratory test could also investigate the function of the heart, especially its endocrine activity, through the family of peptide hormones with potent natriuretic activity such as type B natriuretic peptide (BNP), atrial natriuretic peptide (ANP), type C natriuretic peptide, urodilatin and dendroaspis natriuretic peptide (7).
Usually clinical laboratories concentrate on BNP and N-terminal (NT)-proBNP, which derive from proBNP, the common precursor peptide (1). The European Society of Cardiology Task Force for the diagnosis and treatment of chronic heart failure suggests a close relation between natriuretic peptides levels and heart failure. In fact there is a progressive increase of BNP levels from patients with normal heart function, to patients with preclinical alterations (8) or overt heart failure. Normal levels in untreated patients have a strong negative predictive value for the presence of heart failure and Guidelines recommend testing BNP or NT-proBNP levels in symptomatic patients in order to rule out the diagnosis of heart failure (9).
Laboratory diagnostics
Laboratory diagnostics is commonly defined as the act or process of identifying the nature and cause of a disease by means of in vitro diagnosis testing. Laboratory diagnostics is traditionally divided into three different phases: pre-analytical, analytical and post-analytical. The pre-analytical phase represents the different activities necessary to obtain the biological specimens on which to perform the laboratory evaluations and most of these activities are located outside the clinical laboratory (patients preparation, sample collection, handling and transportation, preparation and storage of the specimens). The leading causes of pre-analytical variability are related to patient preparation, blood drawing, sample transportation and preparation, as reported in Table 1 (10). Several lines of evidence attest that the vast majority of laboratory errors (i.e., approximately 70%), derives from pre-analytical rather than analytical or post-analytical phases (11).
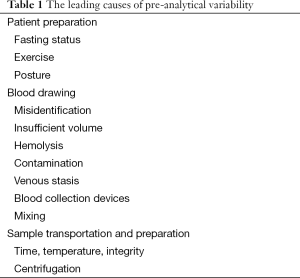
Full table
The receipt of unsuitable sample is relative common in laboratory practice and represents a problem for tests’ quality and results and, consequently, for patients safety. Detection and management of unsuitable samples are necessary to avoid dangerous clinical consequences as recently underlined (12).
Along samples unsuitable for quality (haemolyzed or clotted) and quantity (insufficient or inappropriate volume) (13), additional mishandling practices such as prolonged venous stasis (14), inappropriate mixing of primary blood tubes (15), inappropriate condition of transportation and storage of specimens (16), different conditions of centrifugations like the time (17) and the use or not of centrifuge brake (18) represent a possible cause of inaccuracy of results.
The impact of the blood collection devices (parts of vial, gel separator or tube stopper) on clinical chemistry assay is an intriguing and less known aspect, which can alter laboratory results, as recently reported (19,20).
Even the possible interference of medical contrast media on laboratory testing should be taken into account as an important source of analytical inaccuracy (21,22). All the above mentioned aspects may influence the huge number of tests performed in clinical laboratory.
Patient’s conditions
Patient conditions (i.e., fasting status, exercise, posture and circadian changes) are often an overlooked source of bias in laboratory diagnostics (10).
Fasting status
Lipemia is the second (after hemolysis) most frequent cause of interference in various laboratory methods. Inadequate time of blood sampling after the meal is the most common pre-analytical cause of lipemia and therefore current recommendations entails avoiding foods and beverages for 6-12 hours before testing (23), even if lipemia can be removed in most cases with different techniques like ultracentrifugation or the use of solvents such as LipoClear®. A light meal does not influence the laboratory coagulation tests while significant variation of several clinical chemistry and hematological parameters was described (24-26). At this regard no data are available for cTn, where LipoClear® is not suitable for lipemia removal (27) having shown unacceptable recovery for TnT, according to the desirable specification for imprecision criteria. High speed centrifugation should be used for lipemia removal when proceeding to cardiac troponins’ determination in lipemic specimens (28).
Physical activity
Physical activity is an important pre-analytical variable. Middle and long-term endurance and/or strenuous exercise trigger transient elevations of cardiac biomarkers such cTn, natriuretic peptides and several others biomarkers (29-31). Increased levels of cTnI and cTnT were measured respectively in 30-90% and 10-100% endurance athletes after exercise, being the cut-off for normality more often exceeded when late generation assays were used (30,32,33). Also normal physical activity can induce transient cTn elevation in almost 50% of healthy subjects. These results were obtained with previous generation immunoassay cTnT and cTnI in adolescents after basketball training (34) or treadmill running (35) and confirmed with high-sensitivity immunoassay cTnT after maximal bicycle stress test (36) or recreational resistance training like kettlebell workout (37).
NT-proBNP levels are also affected by vigorous physical activity, resulting much higher in ultramarathon than in half-marathon runners (38,39). No half-marathon runner exhibited NT-proBNP concentrations exceeding the diagnostic thresholds of this assay, whereas values above the cut-off were observed in nearly one-third of ultramarathon runners (38). The substantial raise of NT-proBNP is a clear consequence of the increased myocardiocyte stretch deriving from strenuous aerobic exercise and is barely influenced by plasma volume change or fluid imbalance (38).
In conclusion several lines of evidence show that physical activity is an important source of pre-analytic variability in NT-proBNP and cTn testing, especially when latest generation immunoassay are used. Data about the influence of physical activity on BNP determination are lacking but, being BNP cleaved from the same precursor of NT-proBNP, it seems reasonable that also this biomarker may be influenced by exercise.
A great caution in the evaluation of cardiac biomarker determination shortly after strenuous physical activity is therefore advisable.
Position
Body position during blood collection may induce significant variations of plasma volume, thereby affecting the results of many laboratory tests. cTnT seems to be independent from posture position (supine, seated, standing position) (40), while data on BNP are lacking.
Circadian changes
The cyclic variation showed by many biomarkers (diurnal, monthly, seasonal) is another well-known source of pre-analytical variability. cTnT, measured with a high-sensitivity assay, exhibits a diurnal rhythm, characterized by peak concentrations during the morning hours, gradually decreasing throughout daytime and rising again during nighttime (41), while no data are available for natriuretic peptides.
Age and gender
Using high-sensitivity immunoassay, age and gender-dependent effects have been observed both for cTnI (42,43) and cTnT (44,45). This aspect plays an important role in epidemiological studies and in defining the appropriate cardiac 99th percentile of a reference population for clinical purposes, while it is less affecting natriuretic peptides.
Collection devices
Observations about the role played by collection tubes in the determination of cTnI and T are discordant. Some authors reported lower cTn levels, when measured with no high sensitivity immunoassay in heparin plasma compared with serum, suggesting that the binding of heparin to troponins could decrease their immunoreactivity, depending on the kind of reaction antibody used (46). Another study did not confirm these observations for cTnI, which was shown not to be influenced by the type of collection tubes used (without anticoagulant, with heparin and gel separator and with heparin but without gel separator) (20). Serum and sodium-citrate plasma appeared also to be interchangeable for cTnI measurement (47).
BNP measurement seemed to be highly dependent on type of collection tube, being levels measured on K2-ethylene diamine tetraacetic acid (EDTA) significantly different if compared to lithium heparin with gel separator. Some studies reported an underestimation in BNP measurements performed in K2-EDTA if compared to lithium heparin (48), but other reported an underestimation in measurements performed in heparin and serum samples compared to EDTA (49). Finally other authors suggested heparin plasma as an attractive alternative to the established EDTA samples for the BNP determination (50) or 60%, 39%, 70% and 48% lower results in collection tubes containing citrate, heparin, fluoride and no anticoagulants respectively, compared with samples collected into EDTA tubes (51). The different results obtained in these studies may be due to the different immunoassay used, even if a role of the biological matrix has been also suggested (49). For NT-proBNP determinations EDTA or heparinized plasma samples have been proposed to be indifferently used (52).
Standardization of collection tubes is therefore requested in order to avoid a possible source of results variability.
Haemolysis
Haemolysis is defined as the presence of free haemoglobin concentrations above 0.3 g/L (18.8 mmol/L), which confers a detectable pink to red color to plasma or serum, visible after centrifugation of the specimen (53).
In vitro haemolysis remains the leading cause of unsuitable routine and stat specimens for both outpatient and inpatient samples (54,55). Haemolytic specimens are relative frequent, with a prevalence of 3.3% of all of the routine samples, accounting for 40-70% of all unsuitable specimens.
Haemolysis occurs as a consequence of the release of hemoglobin and other intracellular components from blood cells and may occur both in vivo and in vitro. In vivo haemolysis, due to several pathological conditions, is uncommon and accounts for less than 2% of all haemolytic specimens (56). In vitro haemolysis may be due to problems and difficulty during blood collection, or inappropriate handling, storage or centrifugation of the sample (57).
Interference of haemolysis on laboratory testing might be caused by leakage of haemoglobin and other intracellular components into the surrounding fluid, which could induce false elevations of some analyte, dilution effects, chemical interference of free hemoglobin in a variety of analytic reactions and analyte concentration-dependent spectrophotometric interference (57).
Haemolysis interference is less frequently reported in immunoassay than in photometric assay (58). Interference may occur if the reagent antibodies used in immunoassay are poorly specific and cross-react with some of the compounds released from the erythrocyte. Furthermore, some released compound may bind to the analyte and inhibit the reaction with the antibody (59).
Influence of hemolysis on routine clinical chemistry and coagulation testing has been comprehensively evaluated (60,61). However, reliable information on the potential bias on cardiac marker testing arising from in vitro haemolysis is lacking or, in some case, controversial (62-64).
In our previous study a moderate blood cells lysis, producing a concentration of free haemoglobin up to 0.6 g/L, was not associated to significant clinical difference in the determination accuracy of routine cardiac biomarkers with the analyzer Modular System E (NT-proBNP, cTnT) and Access 2 (cTnI, BNP, Mioglobin). Little or moderate sign of haemolysis in the specimens therefore appears not to significantly affect the determination of cardiac biomarkers, if not high sensitivity cTn assays are used (65). On the contrary, higher levels of haemolysis, biased negatively cTnT testing (66). High sensitivity cTnT showed a negative interference with increasing degrees of hemolysis (67,68). For the current cTnI assay, different degree of interference were shown between different immunoassays, ranging from a positive interference of <1% up to 576% (67).
In conclusion haemolytic specimens may be a variable source of bias in cardiac cTn results. Further studies are needed to evaluate the impact of hemolysis on the very low concentration of the more and more high-sensitivity cTn immunoassay.
Stability
Many studies have analyzed BNP and NT-proBNP stability, but their conclusion are discordant (52,69,70) and, once again, this could depend on the different assays used. Without adding protease inhibitors, BNP stability determined with a fully automated micro-particle enzyme immunoassay using frozen plasma is not reliable (70). Using a chemiluminescence immunoassay, different stability of BNP in relation to its plasma concentration was shown: in frozen plasma BNP remained stable only at physiological levels (71,72), while a pathological levels its concentration decreased over time (71). At room temperature EDTA-plasma BNP declined significantly after 4 h and decreased 2-fold after 48 h of storage (70). Similar significant decrease was shown when BNP was stored at 4 °C (70).
NT-proBNP may be stored at 20 °C for at least 4 months in the absence of protease inhibitors, without a relevant loss of the immunoreactive analyte (70) and, in EDTA-plasma, for up to 48 h at room temperature. Same results were obtained storing NT-proBNP for 6 days at 4 °C or 10 days at 20 °C (52).
Conclusions
Pre-analytic variability is an important aspect that affects the quality of the laboratory results, included cardiac biomarkers determination. In order to correctly evaluate cardiac biomarkers results physicians, especially cardiologists and laboratory specialists, should be aware of all the aspects, which could affect the quality of laboratory results, remembering that pre-analytic variability is an often overlooked significant source of bias.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Clerico A. The increasing impact of laboratory medicine on clinical cardiology. Clin Chem Lab Med 2003;41:871-83. [PubMed]
- Clerico A, Passino C, Franzini M, et al. Cardiac biomarker testing in the clinical laboratory: where do we stand? General overview of the methodology with special emphasis on natriuretic peptides. Clin Chim Acta 2015;443:17-24. [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551-67. [PubMed]
- Jarolim P. High sensitivity cardiac troponin assays in the clinical laboratories. Clin Chem Lab Med 2015;53:635-52. [PubMed]
- Hamm CW, Bassand JP, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999-3054. [PubMed]
- Newby LK, Jesse RL, Babb JD, et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2012;60:2427-63. [PubMed]
- Cowie MR, Mendez GF. BNP and congestive heart failure. Prog Cardiovasc Dis 2002;44:293-321. [PubMed]
- Cemin R, Daves M, Panizza G, et al. Early cardiac risk stratification in obese patients: the need of new simple diagnostic tools. Mediterr J Nutr Metab 2012;5:227-31.
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787-847. [PubMed]
- Lippi G, Mattiuzzi C, Favaloro EJ. Pre-analytical variability and quality of diagnosis testing. Looking at the moon and gazing beyond the finger. N Z J Med Lab Sci 2015;69:4-8.
- Lippi G, Guidi GC, Mattiuzzi C, et al. Preanalytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med 2006;44:358-65. [PubMed]
- Lippi G, Banfi G, Buttarello M, et al. Recommendations for detection and management of unsuitable samples in clinical laboratories. Clin Chem Lab Med 2007;45:728-36. [PubMed]
- Lippi G, Becan-McBride K, Behúlová D, et al. Preanalytical quality improvement: in quality we trust. Clin Chem Lab Med 2013;51:229-41. [PubMed]
- Lima-Oliveira G, Lippi G, Salvagno GL, et al. New ways to deal with known preanalytical issues: use of transilluminator instead of tourniquet for easing vein access and eliminating stasis on clinical biochemistry. Biochem Med (Zagreb) 2011;21:152-9. [PubMed]
- Lippi G, Plebani M. Primary blood tubes mixing: time for updated recommendations. Clin Chem Lab Med 2012;50:599-600. [PubMed]
- Adcock Funk DM, Lippi G, Favaloro EJ. Quality standards for sample processing, transportation, and storage in hemostasis testing. Semin Thromb Hemost 2012;38:576-85. [PubMed]
- Lippi G, Salvagno GL, Montagnana M, et al. Influence of the centrifuge time of primary plasma tubes on routine coagulation testing. Blood Coagul Fibrinolysis 2007;18:525-8. [PubMed]
- Daves M, Giacomuzzi K, Tagnin E, et al. Influence of centrifuge brake on residual platelet count and routine coagulation tests in citrated plasma. Blood Coagul Fibrinolysis 2014;25:292-5. [PubMed]
- Bowen RA, Remaley AT. Interferences from blood collection tube components on clinical chemistry assays. Biochem Med (Zagreb) 2014;24:31-44. [PubMed]
- Daves M, Trevisan D, Cemin R. Different collection tubes in cardiac biomarkers detection. J Clin Lab Anal 2008;22:391-4. [PubMed]
- Lippi G, Daves M, Mattiuzzi C. Interference of medical contrast media on laboratory testing. Biochem Med (Zagreb) 2014;24:80-8. [PubMed]
- Daves M, Lippi G, Cosio G, et al. An unusual case of a primary blood collection tube with floating separator gel. J Clin Lab Anal 2012;26:246-7. [PubMed]
- Simundic AM, Cornes M, Grankvist K, et al. Standardization of collection requirements for fasting samples: for the Working Group on Preanalytical Phase (WG-PA) of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM). Clin Chim Acta 2014;432:33-7. [PubMed]
- Lima-Oliveira G, Salvagno GL, Lippi G, et al. Influence of a regular, standardized meal on clinical chemistry analytes. Ann Lab Med 2012;32:250-6. [PubMed]
- Lima-Oliveira G, Salvagno GL, Lippi G, et al. Could light meal jeopardize laboratory coagulation tests? Biochem Med (Zagreb) 2014;24:343-9. [PubMed]
- Lippi G, Lima-Oliveira G, Salvagno GL, et al. Influence of a light meal on routine haematological tests. Blood Transfus 2010;8:94-9. [PubMed]
- Nikolac N. Lipemia: causes, interference mechanisms, detection and management. Biochem Med (Zagreb) 2014;24:57-67. [PubMed]
- Saracevic A, Nikolac N, Simundic AM. The evaluation and comparison of consecutive high speed centrifugation and LipoClear® reagent for lipemia removal. Clin Biochem 2014;47:309-14. [PubMed]
- Sanchis-Gomar F, Lippi G. Physical activity - an important preanalytical variable. Biochem Med (Zagreb) 2014;24:68-79. [PubMed]
- Scharhag J, Herrmann M, Urhausen A, et al. Independent elevations of N-terminal pro-brain natriuretic peptide and cardiac troponins in endurance athletes after prolonged strenuous exercise. Am Heart J 2005;150:1128-34. [PubMed]
- Nie J, Tong TK, George K, et al. Resting and post-exercise serum biomarkers of cardiac and skeletal muscle damage in adolescent runners. Scand J Med Sci Sports 2011;21:625-9. [PubMed]
- Lippi G, Cervellin G, Banfi G, et al. Cardiac troponins and physical exercise. It’s time to make a point. Biochem Med (Zagreb) 2011;21:55-62. [PubMed]
- Lippi G, Schena F, Salvagno GL, et al. Comparison of conventional and highly-sensitive troponin I measurement in ultra-marathon runners. J Thromb Thrombolysis 2012;33:338-42. [PubMed]
- Nie J, Tong TK, Shi Q, et al. Serum cardiac troponin response in adolescents playing basketball. Int J Sports Med 2008;29:449-52. [PubMed]
- Shave R, Ross P, Low D, et al. Cardiac troponin I is released following high-intensity short-duration exercise in healthy humans. Int J Cardiol 2010;145:337-9. [PubMed]
- Tjora S, Gjestland H, Mordal S, et al. Troponin rise in healthy subjects during exercise test. Int J Cardiol 2011;151:375-6. [PubMed]
- Savukoski T, Mehtälä L, Lindahl B, et al. Elevation of cardiac troponins measured after recreational resistance training. Clin Biochem 2015;48:803-6. [PubMed]
- Salvagno GL, Schena F, Gelati M, et al. The concentration of high-sensitivity troponin I, galectin-3 and NT-proBNP substantially increase after a 60-km ultramarathon. Clin Chem Lab Med 2014;52:267-72. [PubMed]
- Lippi G, Schena F, Salvagno GL, et al. Influence of a half-marathon run on NT-proBNP and troponin T. Clin Lab 2008;54:251-4. [PubMed]
- Lippi G, Salvagno GL, Lima-Oliveira G, et al. Circulating cardiac troponin T is not influenced by postural changes during venous blood collection. Int J Cardiol 2014;177:1076-7. [PubMed]
- Klinkenberg LJ, van Dijk JW, Tan FE, et al. Circulating cardiac troponin T exhibits a diurnal rhythm. J Am Coll Cardiol 2014;63:1788-95. [PubMed]
- Eggers KM, Lind L, Venge P, et al. Factors influencing the 99th percentile of cardiac troponin I evaluated in community-dwelling individuals at 70 and 75 years of age. Clin Chem 2013;59:1068-73. [PubMed]
- Aw TC, Phua SK, Tan SP. Measurement of cardiac troponin I in serum with a new high-sensitivity assay in a large multi-ethnic Asian cohort and the impact of gender. Clin Chim Acta 2013;422:26-8. [PubMed]
- Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem 2012;58:1574-81. [PubMed]
- Franzini M, Lorenzoni V, Masotti S, et al. The calculation of the cardiac troponin T 99th percentile of the reference population is affected by age, gender, and population selection: a multicenter study in Italy. Clin Chim Acta 2015;438:376-81. [PubMed]
- Gerhardt W, Nordin G, Herbert AK, et al. Troponin T and I assays show decreased concentrations in heparin plasma compared with serum: lower recoveries in early than in late phases of myocardial injury. Clin Chem 2000;46:817-21. [PubMed]
- Wang YS, Feng CF, Er TK, et al. Comparison of cardiac troponin I levels in serum and sodium-citrate plasma using the ACCESS 2 immunoassay. Ann Clin Lab Sci 2005;35:453-4. [PubMed]
- Daves M, Pusceddu I, Cemin R. Measurement of Type B natriuretic peptide in heparin and K(2) EDTA plasma. Clin Biochem 2010;43:483-4. [PubMed]
- Lippi G, Fortunato A, Salvagno GL, et al. Influence of sample matrix and storage on BNP measurement on the Bayer Advia Centaur. J Clin Lab Anal 2007;21:293-7. [PubMed]
- Dupuy AM, Terrier N, Dubois M, et al. Heparin plasma sampling as an alternative to EDTA for BNP determination on the Access-Beckman Coulter--effect of storage at -20 degrees C. Clin Lab 2006;52:393-7. [PubMed]
- Wu AH, Packer M, Smith A, et al. Analytical and clinical evaluation of the Bayer ADVIA Centaur automated B-type natriuretic peptide assay in patients with heart failure: a multisite study. Clin Chem 2004;50:867-73. [PubMed]
- Yeo KT, Wu AH, Apple FS, et al. Multicenter evaluation of the Roche NT-proBNP assay and comparison to the Biosite Triage BNP assay. Clin Chim Acta 2003;338:107-15. [PubMed]
- Thomas L. Haemolysis as influence and interference factor. eJIFCC vol 13 no 4. Available online: http://www.ifcc.org/ejifcc/vol13no4/130401002.htm
- Lippi G, Guidi GC. Risk management in the preanalytical phase of laboratory testing. Clin Chem Lab Med 2007;45:720-7. [PubMed]
- Bonini P, Plebani M, Ceriotti F, et al. Errors in laboratory medicine. Clin Chem 2002;48:691-8. [PubMed]
- Carraro P, Servidio G, Plebani M. Hemolyzed specimens: a reason for rejection or a clinical challenge? Clin Chem 2000;46:306-7. [PubMed]
- Lippi G, Blanckaert N, Bonini P, et al. Haemolysis: an overview of the leading cause of unsuitable specimens in clinical laboratories. Clin Chem Lab Med 2008;46:764-72. [PubMed]
- Selby C. Interference in immunoassay. Ann Clin Biochem 1999;36:704-21. [PubMed]
- Wenk RE. Mechanism of interference by hemolysis in immunoassays and requirements for sample quality. Clin Chem 1998;44:2554. [PubMed]
- Lippi G, Salvagno GL, Montagnana M, et al. Influence of hemolysis on routine clinical chemistry testing. Clin Chem Lab Med 2006;44:311-6. [PubMed]
- Lippi G, Montagnana M, Salvagno GL, et al. Interference of blood cell lysis on routine coagulation testing. Arch Pathol Lab Med 2006;130:181-4. [PubMed]
- Lyon ME, Ball CL, Krause RD, et al. Effect of hemolysis on cardiac troponin T determination by the Elecsys 2010 immunoanalyzer. Clin Biochem 2004;37:698-701. [PubMed]
- Sodi R, Darn SM, Davison AS, et al. Mechanism of interference by haemolysis in the cardiac troponin T immunoassay. Ann Clin Biochem 2006;43:49-56. [PubMed]
- Masimasi N, Means RT Jr. Elevated troponin levels associated with hemolysis. Am J Med Sci 2005;330:201-3. [PubMed]
- Daves M, Salvagno GL, Cemin R, et al. Influence of hemolysis on routine laboratory cardiac marker testing. Clin Lab 2012;58:333-6. [PubMed]
- Bais R. The effect of sample hemolysis on cardiac troponin I and T assays. Clin Chem 2010;56:1357-9. [PubMed]
- Florkowski C, Wallace J, Walmsley T, et al. The effect of hemolysis on current troponin assays--a confounding preanalytical variable? Clin Chem 2010;56:1195-7. [PubMed]
- Snyder JA, Rogers MW, King MS, et al. The impact of hemolysis on Ortho-Clinical Diagnostic’s ECi and Roche’s elecsys immunoassay systems. Clin Chim Acta 2004;348:181-7. [PubMed]
- Gobinet-Georges A, Valli N, Filliatre H, et al. Stability of brain natriuretic peptide (BNP) in human whole blood and plasma. Clin Chem Lab Med 2000;38:519-23. [PubMed]
- Mueller T, Gegenhuber A, Dieplinger B, et al. Long-term stability of endogenous B-type natriuretic peptide (BNP) and amino terminal proBNP (NT-proBNP) in frozen plasma samples. Clin Chem Lab Med 2004;42:942-4. [PubMed]
- Daves M, Cemin R. Type B natriuretic peptide stability in frozen plasma. Clin Chem Lab Med 2007;45:1257-8. [PubMed]
- Pereira M, Azevedo A, Severo M, et al. Long-term stability of endogenous B-type natriuretic peptide during storage at -20 degrees C for later measurement with Biosite Triage assay. Clin Biochem 2007;40:1104-7. [PubMed]


