Access and closure of the left ventricular apex: state of play
Introduction
Calcific aortic stenosis is the most frequent manifestation of valvular heart disease. The preferred treatment for patients of all age groups is surgical aortic valve replacement (1). Recently, transcatheter aortic valve implantation (TAVI) has become the standard of care for patients that are deemed to be at high risk for open heart surgery. Efficacy and safety of TAVI in a high-risk population has been confirmed by the randomized, controlled PARTNER trial (2,3).
The most common access route for TAVI is the retrograde transfemoral (TF) approach, followed by the antegrade transapical (TA) approach. Both access routes have distinct indications (4). While the TF route is least invasive and the access of choice at most centers, the apical route is used complementary in patients with poor femoral access.
Whereas TF-TAVI can be conducted percutaneously, TA-TAVI typically requires general anesthesia, surgical exposure of the left-ventricular (LV) apex using a left lateral thoracotomy and apical suturing. Therefore, TA-TAVI is considered to be more invasive than TF-TAVI. Yet, the TA approach holds various benefits such as a short distance from the operator to the annulus, facilitating exact positioning of the valve and the possibility to accommodate larger sheaths. Furthermore, it not only provides direct access to the aortic valve but also the mitral valve allowing for a wide range of interventions (5). Moreover, several studies implicate a potential benefit with a reduced stroke rate in TA-TAVI as compared to TF-TAVI (6).
As many centers follow a “transfemoral first” concept, the TA approach for TAVI is commonly used for patients with contraindications to TF-TAVI, primarily peripheral arterial disease. This pre-selection results in an even higher risk profile of the TA-TAVI patient population (7).
Since the first human TA-TAVI by Ye et al. (8), growing operator experience and constant improvements to devices have fostered a reduction of procedure-related complications (9). Large multi-center studies recently have shown that TA TAVI implantation is safe and reliable with an incidence of less than 1% of procedure-related complications, such as bleeding (10-12).
Nevertheless, if bleeding complications do occur at the apical access site, they have been reported to be associated with a poor prognosis, especially in elderly patients with fragile tissue (13-15). Therefore, reliable hemostasis and secure closure of the left ventricular apex is essential.
By means of further improving safety and ease of the TA approach, various apical closure devices are being developed. Some of these devices are aiming at a completely percutaneous approach with no need for large incisions or rib spreading, thus reducing trauma and post procedural patient discomfort.
Apical closure devices could help in decreasing morbidity and mortality associated with the TA approach, but to date there is sparse clinical data on most of the devices that are currently in clinical use. The aim of this article is to give an overview of current devices for apical closure.
Transapical (TA) access for TA-TAVI devices
The left ventricular apex is considered a versatile access site since it is easily accessible through left lateral mini-thoracotomy and provides direct antegrade access to the aortic as well as retrograde access to the mitral valve.
TA-TAVI of the stenotic aortic valve is a well-standardized procedure using a crimped biological valve on a self-expanding or balloon-expandable stent that is inserted under fluoroscopic guidance and deployed on the beating heart (16). The device that is currently being used most frequently for TA-TAVI is the Edwards SapienTM valve with the AscendraTM delivery system (Edwards Lifesciences, Irvine, CA, USA) (1,17). There is a range of other commercially available valves including the Medtronic EngagerTM (Medtronic, Minneapolis, USA), the JenaValveTM (JenaValve, Munich, Germany), and the Symetis AccurateTM valve (Symetis, Ecublens, Switzerland). The St. Jude PorticoTM valve is currently under investigation for a TA access (St. Jude Medical, Minneaolis, USA).
TA-TAVI procedure
The TA-TAVI procedure is typically performed in a surgical hybrid suite under general anesthesia. The patient is heparinized aiming at an ACT of 300 s. Arterial and venous guidewires are placed into the femoral vessels: a transvenous pacing wire is advanced into the right ventricle and a pigtail catheter is advanced through the femoral artery to the level of the aortic root. These femoral guidewires also serve as a safety net in case of emergency femoral cannulation.
Exposure of the left ventricular apex is established through a left anterolateral mini-thoracotomy along the fifth or sixth intercostal space. Access to the apex is routinely alleviated by a soft tissue retractor. The pericardium is opened whenever possible to determine the localization of the left anterior descending artery. A puncture site is chosen, often slightly lateral and above the real apex, as the myocardial tissue is thicker and more robust there (14,18).
Securing the access site with surgical sutures is a crucial step of the procedure. The sutures are placed paying attention to the left anterior descending artery and are passed through tourniquets to be tightened after sheath removal. Several suture techniques have been described for securing the access site (Figure 1).

The most common technique is the placement of purse-string sutures with Teflon pledgets resulting in a concentrical closure of the access site (Figure 1A).
Prolene purse-string sutures with 4-8 Teflon pledgets arranged in a circle have been described (14,16,19). As an alternative, numerous pledgets with a larger internal diameter of the purse string can be used (16).
An alternative suture technique implies two horizontal mattress sutures (Figure 1B) that are aligned perpendicular to one another (19).
Sipahi et al. proposed an alternative closure technique (Figure 1C) where two deep U-stitches with facing teflon felt stripes are placed resulting in linear closure of the access site (20).
After preparation of the sutures, the left ventricle is punctured with a thin needle. A guide wire is advanced through the aortic valve into the ascending aorta under fluoroscopic guidance. Hereafter a stiff guide wire is placed and balloon aortic valvuloplasty can be performed through a 14-F sheath under rapid ventricular pacing. For deployment of a SapienTM prosthesis, a valve delivery sheath is then placed and the delivery catheter with the crimped prosthesis is advanced into the aortic annulus.
The prosthesis is deployed under rapid ventricular pacing during a period of ventilation arrest. The correct function of the prosthesis is assessed by angiography and transesophageal echocardiography.
By tightening the purse-string sutures during sheath removal the left ventricular apex is closed, and hemostasis is achieved. A short period of rapid ventricular pacing may be used to lower the systolic blood pressure (14).
The pericardium is closed over the apex, thus applying additional compression to the access site. Usually an epicardial pacemaker wire and a left lateral chest tube are placed before standard wound closure. Postoperatively, systolic blood pressure is closely monitored to avoid excess strain on the apex.
Apical closure devices
Various apical closure devices are currently being developed under the premise of increasing overall safety of the TA-TAVI approach by further standardizing the procedure, alleviating left ventricular access and minimizing the risk of complications, such as apical bleeding.
On the one hand, there are concepts relying on the placement of sutures, on the other hand there are suture-based devices and suture-less devices that are implants for apical closure. Table 1 gives an overview of several devices that are already in clinical use or currenty under development.
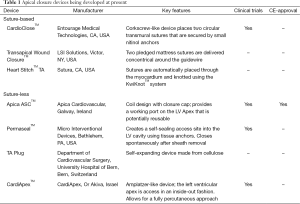
Full table
Suture-based devices
The CardioCloseTM device
The CardioCloseTM device (Entourage Medical Technologies, CA, USA) is a suture-based device that engenders two helical sutures similar to the purse-string technique. The distal end of the device has a wire lumen with two corkscrews, each of which carries a suture and an anchor made of nitinol (Figure 2).
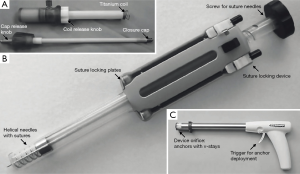
For TA access the left ventricle is punctured, a stiff guide wire is inserted into the left ventricular cavity and the CardioCloseTM system is advanced over a guide wire. Ensuring contact with the epicardium, the screw is turned and sutures are ‘screwed’ into the myocardium until blood flows back into the device. This indicates correct localization of the nitinol anchors in the left ventricular cavity. Upon withdrawal of the CardioCloseTM device the anchors will reside on the endocardium thus securing the two autonomous sutures. Upon sheath removal the sutures can be tightened with suture locking plates to close the access site. The Cardio CloseTM device is being evaluated in first clinical trials. Results are still pending.
Transapical (TA) wound closure system
Knight et al. report on a suture-based automated TA wound closure system (LSI Solutions, Victor, NY, USA) that places two pledgeted mattress sutures concentrically around an apical guide wire (22). The sutures are then crimped together with the Ti-KnotTM device, which deploys mechanical titanium knots. Currently no data regarding clinical trials or first-in-man application is available for this device.
HeartStitchTM TA
The Heart StitchTM TA (Sutura Inc., CA, USA) is a suture-based device that seeks to provide percutaneous closure of the left ventricular apex. Sutures are automatically placed through the myocardium and knotted using the KwiKnotTM system. Currently no data regarding clinical trials or first-in-man application is available for this device.
Suture-less devices
The Apica ASCTM device
The Apica ASCTM device (Apica Cardiovascular Limited, Galway, Ireland) received CE Mark approval in 2013 and is currently in limited commercial launch throughout Europe (23,24). The ASCTM (access, stabilization, closure) device is a pre-mounted suture-less system consisting of three main parts: an introducer, a low-profile titanium coil and a closure cap (Figures 2A,3).

At the beginning of the TA-TAVI procedure the titanium coil is connected to the introducer, which is mounted on the respective THV catheter. Left ventricular access is established by clock wise rotation of the titanium coils into the myocardium while applying constant pressure on the epicardial surface. Altogether, two to three turns are required for safe positioning of the coil (25). The titanium coil then guarantees left ventricular access while performing the TA-TAVI procedure. Subsequent to the procedure, THV catheters and stiff guide wires are removed. The closing cap is then delivered into the titanium coil using the ASCTM introducer device for final closure of the apical access site. It has been noted that significant bleeding from the ASCTM introducer occurs after removal of the valve sheath, necessitating immediate removal of the stiff guide wire and deployment of the closure cap. This might be disadvantageous in case that post-dilatation would be necessary (25). The ASCTM system is suitable for the different sheath sizes of the Edwards Sapien, the Medtronic Engager TM, the JenaValve TM and the Symetis Acurate TM System (25). Since the device is compact in size it might facilitate a non rib-spreading approach with a very small incision. In the future, the access port in the left ventricular apex might potentially be reused in redo-procedures (24).
The PermasealTM device
The PermasealTM device (Micro Interventional Devices, Bethlehem, PA, USA) creates a self-sealing access to the left ventricular cavity by circumferential placement of tissue anchors around the access site that are linked together. The device is currently being evaluated for performance and safety in a European multi-center trial (STASIS, Suture-less Transapical Access and Closure Study) and is likely to receive CE Mark approval soon.
The device looks like a gun and in its first generation bears six anchors that each consist of a solid core and a flexible part that acts as a barb (Figure 2C). Three anchors each are connected by elastic v-stays, so that an operative window of triangular shape is created in the center. Due to their elasticity, v-stays adapt to the size of the inserted sheath and can control devices of up to 28 F.
The current version of the PermasealTM device has been modified to hold eight tissue anchors that are connected with a purse-string suture.
To establish apical access a guide wire is first introduced into the left ventricular access site in a standard manner. The device is fed over the guide wire and approximated to the epicardial surface. When the device is ‘fired’ by pulling the trigger, the anchors penetrate the myocardium. The PermasealTM device is then removed over the wire and the sheath for valve implantation is inserted through the operative window. Once the TA-TAVI procedure is completed, sheath and guide wire can safely be removed during tightening of the purse-string sutures. First clinical experiences at our clinic are promising and so far a safe apical hemostasis has been accomplished in all cases.
A successor of this device that is currently undergoing animal testing will probably allow for total percutaneous access to the left ventricle.
The TA PLUG device
The TA PLUG device (Department of Cardiovascular Surgery, University Hospital of Bern, Switzerland) is a self-expanding and full-core device made from biocompatible cellulose that swells, when in contact with blood. It has a crimped diameter of 27 F and expands up to 66 F. The system is self-anchoring and features integrated proximal and distal radiopaque markers. For deployment the delivery system is fed over the stiff guide wire and the device is placed into the access site under fluoroscopic guidance to align the apex wall within the radiopaque markers. After successful apical sealing, the guide wire is withdrawn (26). To date, there has been no clinical data supporting the feasibility of this device. In the authors’ opinion, sheath removal might be problematic—even if rapid ventricular pacing is applied—because major bleeding from the dilated access site may occur until the device has effectively been deployed.
The CardiApexTM device
The CardiApexTM device (CardiApex Ltd., Or Akiva, Israel) is currently being tested in a European multicenter study. The apex is accessed via the left ventricle in an inside–out fashion and closed with an Amplatzer-like plug device.
A specially designed 9 F catheter is introduced into the left ventricle via TF arterial access and the left ventricular apex is punctured from within. The puncturing-guide wire is being caught percutaneously with a sling and apical access can be established from the outside with the introducer system.
The introducer system consists of an outer cap holding the pericardium in place and an inflatable balloon that stabilizes the access site from within the left ventricle. Following TA-TAVI the apical access site is sealed with a closure-plug resembling an Amplatzer-device. So far no clinical data has been published on the CardiApexTM device. Potentially this device will allow for a purely percutaneous approach without the need for a surgical mini-thoracotomy.
Future directions
In the face of innovations in transcatheter cardiac interventions, apical access and closure devices might be of growing importance as the TA-access is not only feasible for TAVI but also for other catheter-based therapies, such as mitral valve implantation, mitral-valve-chordal insertion, paravalvular leak closure, mitral valve commissurotomy, valve-in-valve procedures of degenerated bioprosthesis in aortic and mitral position and even aortic arch interventions.
Generally speaking, the ideal device for left ventricular access and closure should be eligible for insertion via a mini-skin incision smaller than three centimeters of length with no need for additional rib spreading. Placement of the device should be simple, and it then should comply with the diameter of various instruments passed through the opening. Moreover, the device should leave behind a minimum amount of foreign material.
As devices for a fully percutaneous approach arise, pre-interventional imaging and access-site planning with identification of a safe “puncture window” become even more important. Also, the most suitable intercostal space and the appropriate angulation of the needle should be planned by preoperative computed tomography and fluoroscopic imaging of the left anterior descending artery. It has to be taken into account that in advanced valvular heart disease local anatomy is commonly abnormal with the left atrium often dilated and the apex often rotated upwardly (21). Also, at present, there is no imaging tool that helps predict left ventricular tissue quality. Hence future studies will have to further investigate the use of apical closure devices in patients with fragile left ventricular myocardium. In Europe, to date, only the Apica ASCTM device has received CE-mark. Other devices such as the PermasealTM, the CardioCloseTM and the CardiApexTM device are being evaluated in clinical trials and are expected to receive CE-approval soon.
Conclusions
TAVI has become a standard therapy for the treatment of high-risk patients suffering from severe aortic valve stenosis. A couple of different access options with specific characteristics are available today for transcatheter based valve treatment, and high-volume TAVI programs take advantage of the complementarity of the different access options. The antegrade TA access for TAVI is a safe and well-established strategy with specific advantages such as a short distance from the operator to the aortic valve annulus and the possibility to use large sheath diameters. Additionally, the left ventricular apex not only provides direct access to the aortic valve but also the mitral valve allowing for a wide range of interventions, assigning TA-access a key role in upcoming mitral valve interventions.
The use of apical closure devices is likely to increase overall safety of the TA-TAVI procedure by further standardizing the procedure and minimizing the risk of bleeding from the access site. The ideal apical closure device should be easy to put in place, leave a minimum of foreign material, provide complete hemostasis and have a minimal risk of displacement. So far the range of commercially available devices in Europe is very limited with only one CE-certified device on the market and one device that is expected to receive CE-certification soon. Off-the-shelf closure devices could help flatten the initial operator learning curve and facilitate a safe apical access, ultimately leading to an entirely percutaneous TA-TAVI approach.
Acknowledgements
We want to thank the excellent reviewers for their comments on our manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [PubMed]
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. [PubMed]
- Bleiziffer S, Krane M, Deutsch MA, et al. Which way in? The necessity of multiple approaches to transcatheter valve therapy. Curr Cardiol Rev 2013;9:268-73. [PubMed]
- Shults C, Gunter R, Thourani VH. The versatility of transapical access: Will it lead to a completely new approach to valvular therapy? Ann Cardiothorac Surg 2012;1:220-3. [PubMed]
- Eggebrecht H, Schmermund A, Voigtländer T, et al. Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta-analysis of 10,037 published patients. EuroIntervention 2012;8:129-38. [PubMed]
- Bleiziffer S, Ruge H, Mazzitelli D, et al. Survival after transapical and transfemoral aortic valve implantation: talking about two different patient populations. J Thorac Cardiovasc Surg 2009;138:1073-80. [PubMed]
- Ye J, Cheung A, Lichtenstein SV, et al. Transapical aortic valve implantation in humans. J Thorac Cardiovasc Surg 2006;131:1194-6. [PubMed]
- Généreux P, Webb JG, Svensson LG, et al. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial. J Am Coll Cardiol 2012;60:1043-52. [PubMed]
- Walther T, Thielmann M, Kempfert J, et al. PREVAIL TRANSAPICAL: multicentre trial of transcatheter aortic valve implantation using the newly designed bioprosthesis (SAPIEN-XT) and delivery system (ASCENDRA-II). Eur J Cardiothorac Surg 2012;42:278-83; discussion 283. [PubMed]
- Dewey TM, Bowers B, Thourani VH, et al. Transapical aortic valve replacement for severe aortic stenosis: results from the nonrandomized continued access cohort of the PARTNER trial. Ann Thorac Surg 2013;96:2083-9. [PubMed]
- Wendler O, Walther T, Schroefel H, et al. Transapical aortic valve implantation: mid-term outcome from the SOURCE registry. Eur J Cardiothorac Surg 2013;43:505-11; discussion 511-2. [PubMed]
- Pitta SR, Cabalka AK, Rihal CS. Complications associated with left ventricular puncture. Catheter Cardiovasc Interv 2010;76:993-7. [PubMed]
- Bleiziffer S, Piazza N, Mazzitelli D, et al. Apical-access-related complications associated with trans-catheter aortic valve implantation. Eur J Cardiothorac Surg 2011;40:469-74. [PubMed]
- Généreux P, Cohen DJ, Williams MR, et al. Bleeding complications after surgical aortic valve replacement compared with transcatheter aortic valve replacement: insights from the PARTNER I Trial (Placement of Aortic Transcatheter Valve). J Am Coll Cardiol 2014;63:1100-9. [PubMed]
- Walther T, Dewey T, Borger MA, et al. Transapical aortic valve implantation: step by step. Ann Thorac Surg 2009;87:276-83. [PubMed]
- Thomas M, Schymik G, Walther T, et al. One-year outcomes of cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2011;124:425-33. [PubMed]
- Kempfert J, Treede H, Rastan AJ, et al. Transapical aortic valve implantation using a new self-expandable bioprosthesis (ACURATE TA™): 6-month outcomes. Eur J Cardiothorac Surg 2013;43:52-6; discussion 57. [PubMed]
- Wong DR, Ye J, Cheung A, et al. Technical considerations to avoid pitfalls during transapical aortic valve implantation. J Thorac Cardiovasc Surg 2010;140:196-202. [PubMed]
- Sipahi NF, Papadopoulos N, Moritz A, et al. Linear Closure of the Left Ventricular Apex Following Transcatheter-Based Aortic Valve Implantation. Thorac Cardiovasc Surg 2015;63:508-9. [PubMed]
- Tang GH, Lansman SL, Cohen M, et al. Transcatheter aortic valve replacement: current developments, ongoing issues, future outlook. Cardiol Rev 2013;21:55-76. [PubMed]
- Knight PA, Sauer JS, Kaufer JW, et al. Automated remote transapical wound closure system study. Ann Thorac Surg 2011;92:1494-8. [PubMed]
- Blumenstein J, Van Linden A, Arsalan M, et al. Experimental evaluation of a new apical access and closure device. Ann Thorac Surg 2012;94:1706-9. [PubMed]
- Blumenstein J, Kempfert J, Van Linden A, et al. First-in-man evaluation of the transapical APICA ASC access and closure device: the initial 10 patients. Eur J Cardiothorac Surg 2013;44:1057-62; discussion 1062. [PubMed]
- Conradi L, Seiffert M, Shimamura K, et al. Successful use of a left ventricular apical access and closure device for second-generation transapical aortic valve implantation. Thorac Cardiovasc Surg 2014;62:498-502. [PubMed]
- Brinks H, Nietlispach F, Göber V, et al. Transapical access closure: the TA PLUG device. Interact Cardiovasc Thorac Surg 2013;17:806-9; discussion 809-10. [PubMed]


