Redo aortic valve surgery versus transcatheter valve-in-valve implantation for failing surgical bioprosthetic valves: consecutive patients in a single-center setting
Introduction
During the last two decades a rising trend towards the implantation of bioprosthetic as opposed to mechanical valves has been observed. Therefore, an increase of patients presenting with failing bioprosthetic surgical valves in need of a reoperation is to be expected. Redo surgery may pose a high-risk procedure, especially when considering that many patients are elderly and present with numerous comorbidities (1-3).
Transcatheter aortic valve-in-valve implantation is an innovative, less-invasive treatment for failing bioprostheses in patients at high surgical risk. Numerous studies have shown the feasibility and safety of the valve-in-valve approach (4-8), however a comprehensive evaluation of the outcome in consecutive patients after a valve-in-valve TAVI [transcatheter aortic valve-in-surgical aortic valve (TAV-in-SAV)] as compared to a standard reoperation [surgical aortic valve redo-operation (SAV-in-SAV)] has not yet been performed. We hypothesize, that the less invasiveness including shorter operation time and less surgical trauma, as well as the avoidance of cardio pulmonary bypass (CPB) of the transcatheter approach might have a positive effect on postoperative outcomes in these patients. The goal of this study was to compare postoperative outcomes (including myocardial infarction, stroke, dialysis, pacemaker implantation, transfusion, chest tube output and 30-day mortality) after TAV-in-SAV with SAV-in-SAV in a single center setting.
Methods
Two hundred and ten consecutive patients undergoing isolated redo aortic valve replacement for a failing bioprosthetic valve from January 2001 to October 2014 were retrospectively reviewed. All transcatheter valve-in-valve patients from 2007 to now were also analyzed. Patients with previous mechanical or transcatheter valves, active endocarditis and concomitant cardiac procedures were excluded (Figure 1).
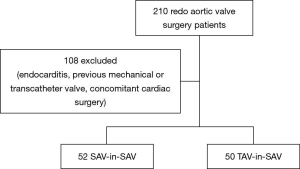
Patient characteristics, preoperative data, post-procedural complications and 30-day mortality were collected from a designated database. Endpoints were reported according to the VARC-2 criteria (9).
All surgical procedures were preformed via a median sternotomy in the usual manner. The transcatheter procedures were planned using CT-guided assessment of access routes and valve measurement. Depending on the access route (transfemoral, transapical or subclavian), the transcatheter procedure varied accordingly. We implanted the Medtronic CoreValve (Medtronic, Inc., Minneapolis, Minnesota), the Edwards Sapien XT and Sapien 3 (Edwards Lifesciences, Irvine, California, USA) and the JenaValve (JenaValve Technology GmbH, Munich, Germany) in transcatheter valve-in-valve procedures. For surgical replacement, the St. Jude Trifecta [St. Jude Medical (SJM), Inc., St. Paul, MN, USA], Medtronic Mosaic, Medtronic Hancock (Medtronic, Inc., Minneapolis, Minnesota, USA), Edwards Perimount, Edwards Perimount magna (Edwards Lifesciences, Irvine, California, USA), St. Jude Regent (SJM, Inc., St. Paul, MN, USA), the Sorin Freedom SOLO and the Sorin Mitroflow were implanted. The decision concerning eligibility for a transcatheter valve-in-valve procedure was made by our multidisciplinary team on the basis of all available clinical and imaging data.
Mean values ± SD were calculated for all continuous variables. Counts and percentages were calculated for categorical variables. The Chi-square and Fisher exact tests were used to compare categorical variables. Continuous variables were compared using the t-test for independent samples. A 2-sided P value <0.05 was considered statistically significant. The intubation time was depicted as a median with the corresponding ranges. Only patients who were not extubated in the operating room (OR) were included. The intubation times were compared using the Mann-Whitney U Test. The Kaplan–Meier method and comparison between the groups was performed using the log-rank statistic. Statistical analysis was performed using IBM SPSS Software Version 22 (Armonk, NY, USA; IBM Corp).
The authors are solely responsible for the design and conduct of this study, all analyses, and its final content.
Results
Between January 2001 and October 2014, 102 patients, who fulfilled the inclusion criteria, underwent isolated redo-aortic valve replacement or transcatheter valve-in-valve implantation for a failing surgical bioprosthesis. Of these, 50 patients (49%) underwent transcatheter aortic implantation and 52 patients (51%) underwent conventional aortic valve surgery (Figure 1).
Baseline data are shown in Table 1. Patients in the TAV-in-SAV group were significantly older, had a higher mean logistic EuroSCORE and exhibited a lower mean left ventricular ejection fraction than patients in the SAV-in-SAV group (78.1±6.7 vs. 66.2±13.1, P<0.001; 27.4±18.7 vs. 14.4±10, P<0.001; and 49.8±13.1 vs. 56.7±15.8, P= 0.019 respectively).
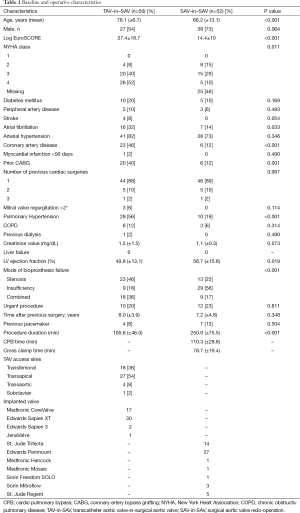
Full table
The type of failing surgical bioprostheses is listed in Table 2. Procedural outcome and postoperative outcomes are listed in Table 3. The rate of postoperative pacemaker implantation and chest tube output were higher in the SAV-in-SAV group compared to the TAV-in-SAV group [11 (21%) vs. 3 (6%), P=0.042 and 0.9±1.0 vs. 0.6±0.9, P=0.047, respectively]. In the TAV-in-SAV group 22 patients (44%) were extubated in the OR. Of the TAV-in-SAV patients requiring further ventilation on the intensive care unit (ICU), there was no significant difference in the median intubation time between the two groups [TAV-in-SAV: 10 h (2-761 h), SAV-in-SAV: 9 h (3-1,008 h), P=0.121].
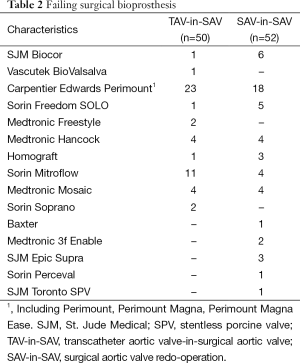
Full table
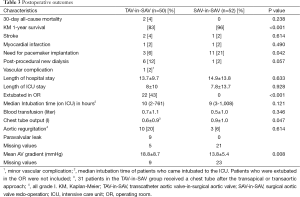
Full table
There was no significant difference for stroke, myocardial infarction and dialysis between the groups.
Thirty-day was also not significantly different between the two groups [TAV-in-SAV: 2 (4%) vs. SAV-in-SAV: 0, P=0.238]. Kaplan-Meier (KM) 1-year survival was significantly lower in the TAV-in-SAV group than in the SAV-in-SAV group (83% vs. 96%, P=0.000).
Discussion
The aim of this study was to compare early outcomes of transcatheter valve-in-valve and redo surgery for the treatment of failing aortic valve bioprostheses in consecutive patients.
Our results suggest that both procedures exhibit good early clinical outcomes. At 30-day follow-up, all-cause mortality was 4% in the TAV-in-SAV group and 0% in the SAV-in-SAV group (NS). Various studies have demonstrated similar or slightly higher mortality rates (7-8.4%) for transcatheter valve-in-valve patients (5,10,11). Mortality rates for SAV-in-SAV are higher (4.5-5.1%) in the literature (1,12), yet these studies included patients with infective endocarditis, which has been identified as a factor for higher mortality (1,13). As patients with endocarditis are not candidates for a transcatheter procedure, we excluded them from our analysis. Our findings are in line with previously reported results between redo aortic valve surgery and transcatheter valve-in-valve procedures in the literature, showing no significant difference in mortality (14-16). In these studies the patient cohorts were matched, and thus, as opposed to our investigation, the patients undergoing redo surgery were at comparable risk to the patients in the transcatheter group. This may explain the higher mortality rates reported for the SAV-in-SAV patients in the literature. In addition, exclusion of endocarditis was not always clarified in these investigations.
Despite a difference in basline characteristics, there was no significant difference in 30-day all-cause mortality, post procedural stroke or myocardial infarction. Similar results were reported by Panchal et al. (15) and by Wilbring et al. (16).
In the present investigation, kidney failure with need for dialysis was not significantly different between the TAV-in-SAV and SAV-in-SAV group. However, a P value of 0.057 suggests a trend towards higher dialysis rates in the TAV-in-SAV group. Our reported dialysis rate of 12% in the TAV-in-SAV group is similar to the rate reported by Wilbring et al. (13.5%) (16) and somewhat higher than the rate reported by Dvir et al. (7.4%) (10). Perhaps the reason for this discrepancy can be found in the cohort size. Wilbring et al. investigated a cohort size similar to ours with 53 patients in each group. In contrast, Dvir et al. analyzed 459 patients of the international registry.
The postprocedural pacemaker rate in the SAV-in-SAV group was significantly higher than in the TAV-in-SAV group (21% vs. 6%).The European RECORD initiative describes slightly lower permanent pacemaker implantation rates of 12.7% (1) for redo surgery patients. Our described pacemaker rates for the TAV-in-SAV group are well in line with previously described results from Dvir et al. (7.4% and 8.3%) (5,10) and Linke et al. (3.7%) (4). This difference in de novo pacemaker implantation rates between SAV-in-SAV patients and TAV-in-SAV patients is well explained by the surgical excision of the previously implanted bioprostheses which implies a respective risk of injury to the conduction system. In contrast to our results, Wilbring et al. found no difference in the postoperative need for a permanent pacemaker between transcatheter valve-in-valve and redo surgery (16). Amazingly, Jones et al. reported even a higher pacemaker implantation rate in the TAVI valve-in-valve group than in the redo surgery group (14). Preoperative ECGs might help to explain these differing outcomes, perhaps showing already existing conduction disturbances.
It is often suggested, that valve-in-valve patients exhibit high postprocedural gardients. In our study, 24% of the TAV-in-SAV patients showed mean gradients >25 mmHg (mean 18.6±8.6 mmHg). Dvir et al. reported similar mean gradients (10). Echocardiographic data was not complete for the SAV-in-SAV patients, yet these showed significantly lower gradients (13.8±5.4 mmHg, P=0.008). Further assessment is needed to verify this result.
Patients after TAVI present more commonly with paravalvular leak, than patients with a surgical valve, who show more often valvular regurgitation. All our patients showed regurgitation grades ≤1 without clinical relevanz.
The higher chest tube output in the SAV-in-SAV group is not surprising, as a redo surgery involves laborious and time-consuming dissection of fibrous tissue and adhesions, causing multiple micro lesions. Yet, this did not result in a higher amount of blood transfusions.
A large number (44%) of the TAV-in-SAV patients were extubated while still in the OR. Those patients who needed further ventilation on the ICU showed no significant difference in the median length of intubation compared to the SAV-in-SAV group. Early extubation may explain, why even with the higher incidence of baseline comorbidities, the length of ICU or hospital stay was not significantly longer in the TAV-in-SAV group. The less invasiveness of the TAV-in-SAV procedure offers great advantages, especially in high-risk patients, allowing a quick postoperative recovery. Thus not only providing a therapy option for previously inoperable patients, but perhaps offering an option for lower-risk patients while exhibiting clinical and economic benefits.
The significant difference in 1-year survival can be explained by the inequality in baseline comorbidities of the groups. Our results are in line with previously reported data (5,10,12).
In conclusion, our study shows that in a small cohort of consecutive patients SAV-in-SAV is associated with low postoperative complications and a low 30-day mortality. On the other hand, patients undergoing TAV-in-SAV showed similar early clinical outcomes as SAV-in-SAV patients, even though they were older and had a higher EuroSCORE. The increased postoperative gradients in the TAV-in-SAV group are of concern and mandate further evaluation as to the optimal type of transcatheter device. The results suggest however, that high-risk patients may profit from the less invasiveness of the transcatheter procedure, keeping in mind that long term data have not been reported yet. Assuming equal durability of transcatheter and bioprosthetic valves and favourable long term data, the results may lead to a shift towards the use of TAV-in-SAV also in lower risk patients.
Limitations
The most important limitation of this study is the lack of matching and randomization to treatment groups. Evaluating these results may thus lead to incorrect conclusions, as the influence of confounding variables may not be clear.
We grouped all TAVI patients together, regardless of the access route. However, 30-day mortality was not significantly different between apical and non-apical access routes (P=0.461).
Postoperative echocardiographic data was not complete for the SAV-in-SAV group. Due to exclusion of certain criteria, our patient groups were relatively small. This may influence certain outcome rates. Conclusions drawn from this small retrospective study have to be critically validated in larger studies. Randomized trials will allow better insights into this topic.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. S. Bleiziffer: Proctor for Medtronic, JenaValve and Boston Scientific; Consultant for Medtronic. Prof. R. Lange: Consultant for Medtronic.
References
- Onorati F, Biancari F, De Feo M, et al. Mid-term results of aortic valve surgery in redo scenarios in the current practice: results from the multicentre European RECORD (REdo Cardiac Operation Research Database) initiative†. Eur J Cardiothorac Surg 2015;47:269-80; discussion 280. [PubMed]
- Maganti M, Rao V, Armstrong S, et al. Redo valvular surgery in elderly patients. Ann Thorac Surg 2009;87:521-5. [PubMed]
- Balsam LB, Grossi EA, Greenhouse DG, et al. Reoperative valve surgery in the elderly: predictors of risk and long-term survival. Ann Thorac Surg 2010;90:1195-200; discussion 1201. [PubMed]
- Linke A, Woitek F, Merx MW, et al. Valve-in-valve implantation of Medtronic CoreValve prosthesis in patients with failing bioprosthetic aortic valves. Circ Cardiovasc Interv 2012;5:689-97. [PubMed]
- Dvir D, Webb J, Brecker S, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation 2012;126:2335-44. [PubMed]
- Bedogni F, Laudisa ML, Pizzocri S, et al. Transcatheter valve-in-valve implantation using Corevalve Revalving System for failed surgical aortic bioprostheses. JACC Cardiovasc Interv 2011;4:1228-34. [PubMed]
- Latib A, Ielasi A, Montorfano M, et al. Transcatheter valve-in-valve implantation with the Edwards SAPIEN in patients with bioprosthetic heart valve failure: the Milan experience. EuroIntervention 2012;7:1275-84. [PubMed]
- Bapat V, Attia R, Redwood S, et al. Use of transcatheter heart valves for a valve-in-valve implantation in patients with degenerated aortic bioprosthesis: technical considerations and results. J Thorac Cardiovasc Surg 2012;144:1372-9; discussion 1379-80. [PubMed]
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403-18. [PubMed]
- Dvir D, Webb JG, Bleiziffer S, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 2014;312:162-70. [PubMed]
- Stähli BE, Reinthaler M, Nguyen-Kim TD, et al. Transcatheter aortic valve-in-valve implantation: clinical outcome as defined by VARC-2 and postprocedural valve dysfunction according to the primary mode of bioprosthesis failure. J Invasive Cardiol 2014;26:542-7. [PubMed]
- Leontyev S, Borger MA, Davierwala P, et al. Redo aortic valve surgery: early and late outcomes. Ann Thorac Surg 2011;91:1120-6. [PubMed]
- Leontyev S, Borger MA, Modi P, et al. Redo aortic valve surgery: Influence of prosthetic valve endocarditis on outcomes. J Thorac Cardiovasc Surg 2011;142:99-105. [PubMed]
- Jones SG, Abdulkareem NR, Williams F, et al. Outcomes of transcatheter aortic valve implantation compared to surgical aortic valve replacement following previous surgery. J Heart Valve Dis 2013;22:204-8. [PubMed]
- Panchal HB, Ladia V, Desai S, et al. A meta-analysis of mortality and major adverse cardiovascular and cerebrovascular events following transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Am J Cardiol 2013;112:850-60. [PubMed]
- Wilbring M, Tugtekin SM, Alexiou K, et al. Transapical transcatheter aortic valve implantation vs conventional aortic valve replacement in high-risk patients with previous cardiac surgery: a propensity-score analysis. Eur J Cardiothorac Surg 2013;44:42-7. [PubMed]


