Clinical manifestations of sleep apnea
Introduction
Obstructive sleep apnea (OSA) manifests in a variety of ways from subtle intrusion into daily life that may be unrecognized by the patient and providers to profound sleepiness, snoring, witnessed apneas and other classic, more recognizable symptoms. Unfortunately the former presentation is the clinical norm. Symptom severity often progresses over years, leading to delayed diagnosis and allowing time for the disease to adversely affect health. Even with increasing awareness of the serious adverse consequences of untreated sleep apnea on patient outcomes and healthcare utilization, epidemiologic studies suggest that OSA is under-diagnosed (1,2). As the obesity epidemic continues to increase, the prevalence of OSA will likely continue to rise. Clinicians should be familiar with both the subtle and overt clinical manifestations of OSA to accurately identify patients at risk for the disease, order appropriate testing, and tailor therapy to the individual patient.
Clinical manifestations
A substantial amount of evidence supports an association between OSA and a number of disorders (3). Healthcare providers should strongly consider the possibility of OSA in patients with these co-morbidities particularly when found in conjunction with characteristic symptoms and physical exam findings of OSA (Table 1). The evaluation of co-morbidities, symptoms, and anatomy is not only important when screening patients for OSA, but is also important with regard to determination of diagnostic testing and treatment modalities.

Full table
Cardiovascular associations
Adverse cardiovascular outcomes are perhaps the most well-described sequela of OSA. Although a number of cardiovascular risk factors (male sex, age, obesity, hypertension, and glucose intolerance) are found frequently in the OSA population, elevated risk is independently associated with OSA. The pathological relationship between sleep apnea and cardiovascular disease has been well-described (4) (Figure 1).
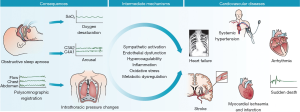
The relationship between OSA and hypertension was first suggested over 30 years ago (5). Subsequently numerous studies have attempted to determine the relationship between OSA and cardiovascular health. Co-morbid hypertension is a common finding in the sleep apnea population (6). The Wisconsin sleep cohort identified a dose-response relationship between sleep apnea severity and incident hypertension at 4 years follow-up independent of known co-founding risk factors (7). Another prospective cohort study reported this dose relationship between OSA and hypertension as well as the impact of continuous positive airway pressure (CPAP) therapy on the development of hypertension (8). This cohort of 1,889 patients without hypertension referred to a sleep center in Spain were divided into three groups: mild, moderate, and severe OSA. They were further classified as being treated with CPAP therapy, non-adherent to CPAP therapy, those who refused CPAP therapy, and those who were ineligible for CPAP therapy. Median follow-up period was 12 years. Incidence of hypertension increased with severity of sleep apnea. The authors found that the hazard ratios (HR) for incident hypertension were greater among patients with OSA ineligible for CPAP therapy [1.33; 95% confidence interval (CI), 1.01-1.75], among those who declined CPAP therapy (1.96; 95% CI, 1.44-2.66), and among those non-adherent to CPAP therapy (1.78; 95% CI, 1.23-2.58). The OSA group adherent to CPAP therapy had the lowest HR (0.71; 95% CI, 0.53-0.94) for the development of hypertension.
Not all studies have confirmed the association of hypertension and OSA. For instance, the Sleep Heart Health Study reported an increased incidence of hypertension with increasing baseline apnea hypopnea index (AHI); however, this finding lost statistical significance after adjustment for baseline BMI (9). The weak association between OSA and systemic hypertension may be related to the older age of the cohort and the inclusion of a very large portion of participants with mild OSA.
A sub-group that deserves special consideration is the population with resistant hypertension, classically defined as individuals requiring three or more antihypertensive medications for blood pressure control. A case series reported by Gonçalves et al. compared 63 patients with resistant hypertension to a control group of 63 patients with controlled hypertension. The prevalence of OSA in patients with resistant hypertension was dramatic at 71% compared with an incidence of 38% in patients with controlled systemic hypertension (10). Recently published work from the Heart BEAT study suggests that severe OSA is associated with a much higher likelihood of persistent elevation in blood pressure in patients on multiple anti-hypertensive medications compared to moderate OSA (11). Based upon the above observations, screening for OSA in patients with resistant hypertension is reasonable; particularly in those with known risk factors for OSA (12,13).
The current evidence base supporting the role of OSA in the development of hypertension is somewhat controversial. Many questions remain unanswered. Treating moderate to severe sleep and symptomatic mild sleep apnea is indicated. Uncertainty arises in the asymptomatic population with mild sleep apnea and comorbid hypertension. The available data suggests that CPAP intervention leads to a modest reduction in blood pressure, and this reduction may be more prominent in the severe OSA population (14). Based on the modest effect CPAP therapy would be expected to have in the mild OSA population, the impact of introducing this therapy in mild disease is likely minimal. Long-term follow-up data assessing the interaction of OSA, hypertension, and cardiovascular morbidity is lacking.
Altogether the evidence supports an association between OSA and the development of hypertension, particularly in more severe disease. It is likely that a range of individual susceptibility exists for the development of hypertension among patients at a given severity of OSA (15). The available data suggests improvement (albeit modest) in blood pressure with CPAP therapy; however, the introduction of CPAP therapy in a patient with mild sleep apnea, who is asymptomatic, is unlikely to have a clinically significant impact.
Arrhythmias, particularly atrial fibrillation, have been well-documented in the OSA population and are likely consequences of respiratory abnormalities and changes in autonomic tone during sleep. Contemporary case-control and cross-sectional studies as well as studies dating back 30 plus years support the relationship between OSA and cardiac arrhythmia (16,17). The rhythm disturbances documented in these studies include ventricular tachycardia or fibrillation, sinus arrest, atrioventricular conduction blocks, complex ventricular ectopy, supraventricular tachycardia, bradycardia, and atrial fibrillation (16-21).
Practitioners should be aware of the prevalence of OSA in patients with atrial fibrillation not only for diagnostic purposes, but also for potential treatment implications. Epidemiological evidence for this association is supported by Mehra et al. analysis of the Sleep Heart Health Study data that reported the likelihood of atrial fibrillation is increased 4- to 5-fold in patients with sleep disordered breathing (SDB) (22). Other studies indicate that the risk of atrial fibrillation rises as the severity of sleep apnea increases (23,24). Evidence also suggests that treatment of sleep apnea decreases the risk of recurrence of atrial fibrillation following cardioversion or pulmonary vein isolation procedures (25-27). Kanagala and colleagues conducted a prospective observational study of 39 participants with OSA following cardioversion and the relationship between CPAP usage and atrial fibrillation/flutter recurrence (25). Twenty-seven of these patients were not on positive airway pressure therapy or had suboptimal adherence. Recurrence of AF at 12 months in these 27 patients was 82%, significantly higher than the 42% recurrence in the treated OSA group (n=12, P=0.013). Follow-up data were also obtained in 79 randomly selected post-cardioversion patients (controls) who did not have any previous sleep study; this group had a 53% recurrence of atrial fibrillation at 1 year (P=0.009). A recent study identified 62 patients referred for pulmonary vein isolation for treatment of atrial fibrillation who had sleep apnea confirmed by polysomnography (27). These participants were divided into a “CPAP user” group (n=32) and a “CPAP non-user” group (n=30). The CPAP user group had a lower rate of recurrent atrial fibrillation (28% vs. 63% for CPAP users vs. non-users; P=0.01). Also, a higher arrhythmia-free survival rate off antiarrhythmic drugs (66% vs. 33%; P=0.02) was reported.
Overall, the data suggests a strong relationship between atrial fibrillation and OSA. There is also some suggestion that patients undergoing attempts to re-establish normal sinus rhythm will have more success if their OSA is corrected with positive airway pressure therapy; however this is based on small observational trials and further study is needed.
SDB is frequently encountered in the heart failure population and may manifest as pure OSA or central apnea with Cheynne-Stokes respiration. This association was reported by Javaheri in 1998 when he evaluated 81 ambulatory men with congestive heart failure (CHF) (defined as left ventricular ejection fraction <45%) who agreed to undergo screening polysomnography (28). SDB (defined as an AHI ≥15 events per hour) was present in 51% of these individuals with a predominance of central sleep apnea in this cohort. A larger study undertaken by Sin and colleagues attempted to both better define the prevalence of SDB in the heart failure population and identify risk factors associated with the development of central and/or obstructive apnea in this population (29). A total of 450 participants were studied and, using a cut-off AHI of ten events per hour of sleep, 72% were identified as having SDB (prevalence was 61% and 53% for AHI cutoffs of 15 and 20 respectively). Of the 72% of participants identified with SDB, 148 had central sleep apnea; 168 had obstructive apnea; and 134 patients did not show evidence of SDB. Risk factors for the presence of central sleep apnea included male sex, hypocapnia during wakefulness, atrial fibrillation, and age >60 years old. Risk for OSA was increased in women >60 years old and was increased in men who had a BMI of >35 kg/m2. SDB appears to be very common in the heart failure population with larger studies reporting a prevalence of at least 50%, even in patients who are optimally managed (30).
CHF and SDB seem to have a bi-directional relationship with one leading to and worsening the other. Kaneko et al. reported that CPAP therapy for OSA improves cardiovascular parameters including blood pressure and left ventricular systolic function in participants with CHF supporting the notion that obstructive apnea may represent a modifiable factor in the care of these types of patients (31). Support for the benefit of these physiologic improvements leading to clinically significant outcomes comes from Japan. Investigators followed 88 patients with moderate-to-severe sleep apnea with co-morbid heart failure who were randomized to CPAP therapy (n=65) and no therapy (n=23) (32). The CPAP therapy group was further subdivided into participants who were adherent and those who were non-adherent. The risk for death and hospitalization was increased in the untreated group (HR 2.03; 95% CI, 1.07-3.68; P=0.030) and in less compliant CPAP-treated patients (HR 4.02; 95% CI, 1.33-12.2; P=0.014). CPAP therapy appears to improve outcomes in patients with CHF, provided they are adherent to therapy.
Pulmonary hypertension (PH) is another cardiovascular disease with a well-established relationship to OSA. This association led to inclusion of OSA into group 3 (PH due to lung diseases and/or hypoxia) of the World Health Organization’s PH classification system (33). PH in this population is typically only encountered in moderate-to-severe OSA and is generally mild. In one study, participants (n=220) referred for polysomnography were found to have moderate-to-severe OSAS (AHI >20) and agreed to right heart catheterization (34). Thirty-seven participants were found to have PH defined as a resting mean pulmonary artery pressure (PAP) of at least 20 mmHg. Only 2 of these 37 participants had a mean PAP greater than 35 mmHg. Those with PH tended to have more obstructive lung disease, more severe sleep apnea, a higher arterial carbon dioxide tension, a lower arterial oxygen tension, and a higher BMI. Two subsequent studies evaluating participants with at least mild OSA and without COPD found approximately 20% had PH generally in the mild range (35,36).
Given the mild increase in pulmonary arterial pressure from OSA, one would expect that therapy in OSA patients with positive airway pressure would lead to only a modest reduction in PAP. Reports from 1970s and 1980s suggested improvement in PH with tracheostomy for OSA (37,38). The impact of contemporary management options for OSA such as positive airway pressure and oral appliance therapy in PH is not well studied. Arias and colleagues have published the only randomized control trial to date that has evaluated the impact of CPAP therapy on PH in OSA (39). They studied 23 participants in a crossover design with overall severe OSA (mean AHI 44 events per hour). Participants were randomized to sham CPAP or CPAP therapy and followed for 12 weeks. Baseline echocardiography was performed and then followed up after the treatment period. Ten out of 23 patients had PH at baseline. Two participants were excluded from the study because of CPAP non-adherence. Effective CPAP was associated with a significant although modest reduction in the values for pulmonary systolic pressure from 29 to 24 mmHg.
The recognition of adverse cardiovascular outcomes in patients with SDB is important not only due to the significant burden cardiovascular disease places on society, but also because effective treatment for OSA seems to curtail the cardiovascular risk in these patients. As described above, OSA is associated with many cardiovascular co-morbidities including hypertension, arrhythmias, CHF, and pulmonary HTN. The development of coronary artery disease may also be influenced by the presence of OSA (40). To explore the benefit of therapy, Marin et al. followed 277 age-matched and BMI-matched healthy men, 389 men with simple snorers, 409 patients with mild-moderate OSA, and 667 patients with severe OSA. The OSA group was further sub-divided into patients who used PAP therapy and those who did not use PAP (41). Their results revealed that the incidence of fatal and non-fatal cardiovascular events in untreated patients with severe OSAS was significantly higher than in healthy participants recruited from the general population matched for age and BMI. Importantly, treatment with CPAP significantly reduced cardiovascular risk in patients with severe OSAS.
Neurocognitive associations
Perhaps that best described neurologic sequela that also overlaps with cardiovascular consequences of OSA is stroke. Much work has been undertaken to understand the risk OSA portends for stroke. Other researchers have endeavored to study patients with SDB as a consequence of stroke.
The previously mentioned cardiovascular consequences of OSA, including atrial fibrillation, heart failure, and hypertension, all can lead to stroke. However, OSA in itself seems to lead to an increased risk for stroke independent of these other factors. Data from an observational cohort study of 1,022 patients referred for polysomnography found that OSA lead to a statistically significant increase in the risk of stroke or death (HR 1.97; 95% CI, 1.12-3.48; P=0.01) after adjustment for age, sex, race, smoking status, alcohol-consumption status, body-mass index, and the presence or absence of diabetes mellitus, hyperlipidemia, atrial fibrillation, and hypertension (42). Cross-sectional data from the Sleep Heart Health Study suggests patients with a higher AHI are at an increased risk for stroke. A subsequent report by Shahar et al. created four equal size groups (quartiles) for each index of sleep-disordered breathing using its percentile distribution in the sample (40). Participants in the highest quartile were found to have a 1.58 times greater odds for stroke compared to patients in the lowest quartile. A more recent analysis of prospective data from the Sleep Heart Health Study suggests that severe OSA is an independent risk factor in stroke for men (43).
OSA appears not only to be a risk factor stroke, but also seems to be highly prevalent in the post-cerebrovascular accident (CVA) population. Findings from a 2010 meta-analysis of 29 articles involving over 2,300 patients with stroke (ischemic, hemorrhagic or TIA) are presented below (44). The incidence of SDB with an AHI >5 was 72% and with an AHI >20 was 38%. A CNS insult is a known risk factor for central apnea, yet only 7% of the post-stroke patients with SDB had primarily central apnea in this analysis.
Treatment of SDB in patients whom have suffered a stroke may improve clinical outcomes. A prospective observational study involving 166 participants with stroke who subsequently underwent a sleep study reported that treatment of OSA improved mortality (45). Of these 166 individuals, 96 who had an AHI of 20 or greater were offered CPAP therapy. Participants with an AHI of 20 or greater and intolerant of CPAP (n=68) had an increased risk of mortality (HR 2.69; 95% CI, 1.32-5.61) compared with participants with an AHI of less than 20 (n=70). Individuals intolerant of CPAP therapy were also found to have an increased risk of mortality (HR 1.58; 95% CI, 1.01-2.49; P=0.04) when compared with participants with moderate to severe OSA who tolerated CPAP (n=28). No differences in mortality were identified among participants without OSA, those with mild disease, and those who were adherent to CPAP therapy. Immediate intervention for OSA in patients with stroke may impact short-term outcomes such as stroke severity and functional outcomes. Bravata et al. reported a population of 55 participants randomized to receive auto-CPAP therapy (n=31) within 2 days of stroke and a control group who received usual care (n=24) (46). Only those with polysomnorgraphic evidence of sleep apnea were continued on a full 30 days of auto-CPAP therapy. Individuals randomized to auto-CPAP had greater improvements in NIH Stroke Scale at 30 days (−3.0) compared to the control group (−1.0); P=0.03. Greater improvement was observed with increasing auto-CPAP use. Another small study evaluated introducing PAP therapy in patients with stroke undergoing inpatient rehabilitation (47). Participants were screened with PSG and 48 were identified to have significant sleep apnea (AHI ≥15). A total of 22 participants completed the study with CPAP and another 22 completed the standard care arm. CPAP was found to be of substantial benefit in terms of stroke recovery, functional and motor outcomes, and depression severity. CPAP therapy appeared to have minimal impact on cognitive outcomes.
The evidence that introduction of CPAP therapy to treat OSA improves stroke-specific outcomes remains small and mixed. Although the above studies suggest benefit of early CPAP intervention for patients with stroke and OSA, other small and similarly-designed studies failed to show significant impact of CPAP therapy on outcomes (48,49). To date, long-term data is lacking. Large randomized trials are needed to further assess the impact of treating OSA in patients with stroke.
OSA is associated with variable degrees of neurocognitive impairment. Traffic accidents and work performance deficits are surrogate markers of these neurocognitive impairments in the OSA population that may be reported in clinic. Antonelli et al. found that 1 in 4 patients with newly diagnosed OSAS had severe neurocognitive impairment, particularly with regard to inductive and deductive thinking as well as constructive ability (50). Beebe and colleagues performed a meta-analysis of 1,092 patients with OSAS. Their results suggested a substantial impact of OSA upon vigilance and executive functioning (51). A number of small studies have evaluated various domains of neurocognition and pooled data suggest OSAS has negative effects on inductive and deductive reasoning, attention, vigilance, learning, and memory (52,53). Not all patients with OSA develop neurocognitive impairment and risk factors for development have been described (52). As with the other described clinical manifestations of OSA, treatment of SDB may ameliorate the neurocognitive consequences of this disease (53).
There also seems to be a bi-directional between sleep health and mood disorders. For instance, a prospective cohort study by Peppard included 1,408 patients and found a 1.8 fold risk for the development of depression in patients with OSA (54). Conversely those with baseline depression had a 1.6 fold risk for the development of OSA. This longitudinal study also found a dose-response association between OSA and depression, suggesting a possible a causal link between these conditions. Other studies have not found a relationship between depression and OSA with a link between the two seeming particularly weak in men (55).
Despite the lack of definitive data confirming a relationship between OSA and depression, one disease may impact the other. A case series (n=17) explored the relationship between OSA and persistent major depression despite aggressive pharmacotherapy (56). Beck Depression Inventory (BDI) and Hamilton Rating Scale for Depression (HRSD) scores decreased significantly from 19.7 to 10.8 and 16.7 to 8.0 after 2 months of CPAP treatment (both P<0.01). These participants had severe OSAS at baseline with a mean pre-treatment AHI of 52. Further, there was a significant reduction in residual depressive symptoms, as measured by BDI and HRSD, and in subjective daytime sleepiness reflected in the Epworth score. These findings suggest improvement in residual depressive symptoms may correlate with the improvement of daytime sleepiness by correcting the underlying apnea in these patients. Another trial found not only that treating OSA with CPAP improved depression symptoms, but also the magnitude of improvement of depression symptoms correlated with the degree of improvement CPAP had on OSA (57). Depression also appears to modify the clinical course of OSA. In a cross-sectional study of participants with untreated OSA (n=56), participants were asked to complete the Center for Epidemiologic Studies Depression (CESD), Profile of Mood States (POMS) and Medical Outcomes Studies (MOS) surveys (58). Hierarchical linear regression was applied to the data and OSA severity explained 13.4% (P=0.022) of variance while depression scores explained an additional 24.5% of fatigue. These results suggest that depressive symptoms are of greater importance than OSA severity indices in explaining fatigue in patients with these comorbid diseases.
OSA-induced cognitive insults (manifested by impaired judgment, slowed reaction time, impaired learning, and poor working memory) may compromise driving and performance at work (59). Patients with OSA often complain of difficulty staying on task at work, falling asleep inappropriately at their work space, memory trouble causing professional difficulties, and administrative disciplinary action related to poor performance. Neurocognitive insult from OSA is particularly worrisome in driving, specifically in professional drivers in whom lapses may have dire consequences. The estimated increased risk for a motor vehicle accident in OSA population is 2-7 times that of the unaffected population (60). Data also suggests that effective intervention with positive airway pressure therapy leads to a return to baseline driving capabilities (61); however, the overall risk for a motor vehicle accident in an individual patient is more difficult to assess (62). The official ATS clinical practice guideline suggests risk stratification of drivers, and states that “there is no compelling evidence to restrict driving privileges in patients with sleep apnea if there has not been a motor vehicle crash or an equivalent event” (62). The guideline defines a high-risk driver as a patient with OSA and significant daytime sleepiness, a recent motor vehicle crash or near miss accident due to sleepiness, fatigue, or in-attention (62). The best approach to driving status in the non-sleepy patient with OSA is yet to be determined.
Cerebrovascular insult and impaired neurocognition are commonly associated with OSA. Therapy for OSA may ameliorate atherosclerotic progression and improve outcomes post-CVA. OSA should be considered in patients complaining of poor concentration at work, actual or near-miss motor vehicle accidents, and patients with severe sleepiness as a component of their co-morbid mood disorder.
Metabolic associations
Evidence to date suggests that OSA has an adverse effect on individuals’ metabolic profile, particularly in moderate-to-severe disease (63). The adverse impact of the OSA on glucose homeostasis, lipid metabolism, and fatty liver disease suggests that OSA should be considered as a potential component of metabolic syndrome (64,65).
Glucose metabolism is impacted by the presence of OSA. Alterations in the hypothalamic-pituitary-adrenal axis, aberrant sympathetic activation, induction of certain adipokines, and increased inflammation/oxidative stress caused by SDB have been identified as the intermediary steps between OSA and altered glucose metabolism (66). Investigators at Johns Hopkins used the frequently sampled intravenous glucose tolerance test in 118 non-diabetic participants with and without SDB to model the in vivo kinetics of glucose and insulin (67). They found a significant reduction in insulin sensitivity in patients with OSA, and this reduction in insulin sensitivity correlated with severity of SDB. These findings were independent of age, sex, race, and percent body fat.
Because physiologic abnormalities inherent in OSA are known to alter glucose metabolism, debate remains over the relationship between OSA and diabetes. . In a cross-sectional study of the Wisconsin Sleep Cohort, the odds ratio for having a physician diagnoses of diabetes mellitus with an AHI of 15 or greater versus an AHI of less than 5 was 2.30 (95% CI, 1.28-4.11; P=0.005) after adjustment for age, sex, and body habitus (68). However, these investigators did not find a statistically significant independent causal effect for the development of type II diabetes in their prospective analysis. Also, the incidence of diabetes over a 4-year follow-up period was not significantly related to the severity of SDB at the time of initial enrollment in the cohort when shared risk factors were taken into consideration. Taking the opposite approach to the Wisconsin Cohort, investigators analyzing data from the Sleep Heart Health study assessed the prevalence of OSA in patients with type 2 diabetes (69). Descriptive analyses revealed differences between diabetic and non-diabetic participants in respiratory disturbance index (RDI) (RDI ≥15 prevalence 24% in diabetic cohort versus 15.6% in non-diabetic cohort), as well as sleep stages, sleep time < 90% O2 saturation, central apnea index, and periodic breathing (P<0.05, all). However, multivariable regression analyses that adjusted for age, sex, BMI, race, and neck circumference eliminated these differences for all sleep measures except percent time in rapid eye movement (REM) sleep. Most studies to date have found an increased prevalence of diabetes in the OSA population; however, any elevated risk is typically nullified when adjusting for known cofounders. A recent cross-sectional analysis of 6,616 participants found an increased risk of diabetes in patients with OSA even after controlling for cofounders. Investigators applied multivariate regression analysis to assess type two diabetes mellitus prevalence according to OSA severity, as measured by the oxyhemoglobin desaturation index (70). Results from this evaluation found that the prevalence of type 2 diabetes increased with severity of OSA (6.6% in participants without OSA compared to 28.9% in those with severe OSA). Further this increased prevalence persisted in patients with mild, moderate, and severe OSA compared to the group without OSA after adjustment for cofounders [OR (95% CI) of 1.33 (1.04-1.72), 1.73 (1.33-2.25), and 1.87 (1.45-2.42) (P<0.001) respectively].Whether OSA has detrimental effects on diabetes risk remains unclear, but the high prevalence of diabetes in the OSA population is difficult to ignore.
Correction of apneic events with positive airway pressure seems to modestly improve glycemic control. One of the largest studies by Harsch et al. attempted to assess the impact of CPAP therapy on insulin sensitivity (71). Forty non-diabetic participants (AHI >20) were treated with CPAP. Prior to initiation of CPAP, 2 days after, and after 3 months of effective CPAP treatment, hyperinsulinemic euglycemic clamp studies were performed to evaluate insulin sensitivity. Increased insulin sensitivity occurred after 2 days and remained stable after 2 months. Improvement in insulin sensitivity was most pronounced in the non-obese (BMI <30 kg/m2) participants suggesting that obesity plays a larger role in insulin resistance than apnea. Based on these results, however, non-obese patients may expect a more dramatic increase in insulin sensitivity following CPAP therapy. Two observational trials involving patients with moderate-to-severe OSA suggested modest improvements in HbA1C levels (~0.2% decrease in both studies) following 3-5 months of CPAP therapy (72,73).Improvement in insulin sensitivity and glycemic control with correction of apnea via positive airway pressure therapy has not been universally confirmed, but overall positive airway pressure does appear to have some impact on glycemic control; particularly in those with severe OSA (74,75).
OSA appears to have an impact on lipid metabolism with studies suggesting functional abnormalities in high-density lipoproteins (HDL) and elevations in total cholesterol, low-density lipoproteins (LDL), and triglyceride levels (65,76,77). Compared to the other clinical abnormalities associated with OSA, much less data exists regarding this association. The pathobiology is just beginning to be understood and current data implicates OSA in decreased lipoprotein clearance, increased lipolysis, and enhanced hepatic lipid output (78). Further support for an interaction between OSA and lipid metabolism comes from an observed relationship between severity of desaturation and triglyceride and LDL levels in a murine model (79). Nominal data also exists linking CPAP therapy with improvement in dyslipidemia. A study in Greece evaluated serum risk factors at baseline and 6 months in 53 participants newly diagnosed with OSA (80). They were non-smokers with no comorbidities or medication use. These serum cardiovascular risk factors included high-sensitivity C-reactive protein, homocysteine, total cholesterol, triglycerides, HDL, and LDL. These participants were classified into three groups: group 1 (n=20), good compliance (≥4 h use per night); group 2 (n=19), poor compliance (<4 h use per night); and group 3 (n=14), refusal of CPAP treatment. Group 1 had significant decreases in hs-CRP (P=0.03), homocysteine (P=0.005), total cholesterol (P=0.021), and total cholesterol/HDL-C ratio (P=0.018). Group 2 subjects showed a decrease in homocysteine levels (P=0.021) only. There were no significant changes from baseline in group 3.
Recent interpretation of the available data has led to the suggestion that OSA is yet another constituent of the metabolic syndrome. Adult Treatment Panel III defines the metabolic syndrome as the presence of any three of the following five traits: (I) abdominal obesity; (II) elevated serum triglycerides or drug treatment for elevated triglycerides; (III) low serum HDL cholesterol; (IV) hypertension or drug treatment for elevated blood pressure; and (V) elevation in fasting plasma glucose or drug treatment for elevated blood glucose (81). The International Diabetes Federation (IDF) updated their metabolic syndrome criteria in 2006 with central obesity as an essential element in their definition (82). The correlation between OSA and visceral fat deposition has been well described. Work by Vgontzas et al. analyzed the fat deposition of 14 obese participants with OSAS and ten obese participants without OSA (83). No differences between the two groups in terms of total body fat or subcutaneous fat were identified. The OSA group compared to obese controls had a significantly greater amount of visceral fat (P<0.05 at levels measured). Further, BMI and the amount of subcutaneous fat did not correlate with metrics of OSA severity, whereas visceral obesity was significantly correlated with increasing AHI and degree of nocturnal hypoxemia. The fundamental role of central obesity in OSA and metabolic syndrome as well as the previously discussed associations between OSA and the other defining traits of the metabolic syndrome (hypertension, dyslipidemia, and hyperglycemia) suggests a possible common genetic determinant of this phenotype. There is currently inadequate data from humans, due to the inherent challenges of separating out effects of SDB and obesity, to completely understand the independent impact OSA has on metabolism.
Liver steatosis is a known manifestation of insulin resistance and metabolic syndrome. Recent investigations into the impact OSA has on individuals with the metabolic syndrome and the progression from asymptomatic hepatic steatosis, nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), and finally cirrhotic liver disease have been undertaken. Unfortunately a paucity of data currently exists, but the data pool is expanding.Human studies are limited by less invasive evaluations of the liver (imaging, liver enzymes) and do not distinguish between hepatic steatosis, NAFLD, and NASH. Most histopathologic data come from OSA patients with severe morbid obesity that undergoes a liver biopsy during bariatric surgery (84). OSA may lead to hepatic steatosis. A study by Tanné et al. evaluated liver damage in 163 consecutive nondrinking patients who had undergone polysomnographic recording for clinical suspicion of OSA (85). Liver enzymes were obtained in all patients, and liver biopsy was offered to patients with elevated liver enzymes. Participants with severe OSA (defined as an AHI >50 events per hour) had a higher percentage of steatosis as well as liver necrosis and fibrosis, despite having a similar BMI. Other small studies have not demonstrated an association between OSAS and hepatic steatosis (86,87). Although a study of 40 obese Japanese men with sleep apnea reported that an intervention with nasal CPAP to correct SDB ameliorated liver injury, independent of changes in adiposity (88).
OSA screening
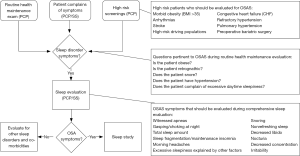
As outlined above OSA can have significant health consequences. Appropriate screening for OSA by primary care providers or sleep specialist is essential to establishing a diagnosis and initiating appropriate treatment. Health care providers screening patients in clinic should take into consideration the classic signs and symptoms as well as high risk populations as outlined in Figure 2.
Conclusions
OSA can present in a variety of ways. OSA can significantly impact co-morbid disease leading to worse outcomes in this patient population. Providers should be vigilant of some the more subtle clinical manifestations of OSA. The cardiovascular, neurocognitive, and metabolic manifestations and can have a significant impact on patient health and quality of life Evidence exists that therapy not only improves outcomes in general, but modifies the severity of co-morbid disease as well. As the obesity epidemic increases, providers will be faced with more patients suffering from OSA. To mitigate the long-term sequela of this disease, providers should be prepared to order appropriate screening tests and provide proper longitudinal management.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997;20:705-6. [PubMed]
- Colten HR, Altevogt BM, editors. Sleep Disorders and Sleep Deprivation. Washington (DC): National Academies Press (US), 2006.
- Mannarino MR, Di Filippo F, Pirro M. Obstructive sleep apnea syndrome. Eur J Intern Med 2012;23:586-93. [PubMed]
- Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation 2012;126:1495-510. [PubMed]
- Tilkian AG, Guilleminault C, Schroeder JS, et al. Hemodynamics in sleep-induced apnea. Studies during wakefulness and sleep. Ann Intern Med 1976;85:714-9. [PubMed]
- Fletcher EC, DeBehnke RD, Lovoi MS, et al. Undiagnosed sleep apnea in patients with essential hypertension. Ann Intern Med 1985;103:190-5. [PubMed]
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378-84. [PubMed]
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012;307:2169-76. [PubMed]
- O'Connor GT, Caffo B, Newman AB, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med 2009;179:1159-64. [PubMed]
- Gonçalves SC, Martinez D, Gus M, et al. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest 2007;132:1858-62. [PubMed]
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014;10:835-43. [PubMed]
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001;19:2271-7. [PubMed]
- Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, et al. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest 2007;131:453-9. [PubMed]
- Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med 2007;167:757-64. [PubMed]
- Mohsenin V. Obstructive sleep apnea and hypertension: a critical review. Curr Hypertens Rep 2014;16:482. [PubMed]
- Tilkian AG, Guilleminault C, Schroeder JS, et al. Sleep-induced apnea syndrome. Prevalence of cardiac arrhythmias and their reversal after tracheostomy. Am J Med 1977;63:348-58. [PubMed]
- Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol 1983;52:490-4. [PubMed]
- Harbison J, O'Reilly P, McNicholas WT. Cardiac rhythm disturbances in the obstructive sleep apnea syndrome: effects of nasal continuous positive airway pressure therapy. Chest 2000;118:591-5. [PubMed]
- Zwillich C, Devlin T, White D, et al. Bradycardia during sleep apnea. Characteristics and mechanism. J Clin Invest 1982;69:1286-92. [PubMed]
- Becker HF, Koehler U, Stammnitz A, et al. Heart block in patients with sleep apnoea. Thorax 1998;53 Suppl 3:S29-32. [PubMed]
- Namtvedt SK, Randby A, Einvik G, et al. Cardiac arrhythmias in obstructive sleep apnea (from the Akershus Sleep Apnea Project). Am J Cardiol 2011;108:1141-6. [PubMed]
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med 2006;173:910-6. [PubMed]
- Hoffstein V, Mateika S. Cardiac arrhythmias, snoring, and sleep apnea. Chest 1994;106:466-71. [PubMed]
- Tanigawa T, Yamagishi K, Sakurai S, et al. Arterial oxygen desaturation during sleep and atrial fibrillation. Heart 2006;92:1854-5. [PubMed]
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003;107:2589-94. [PubMed]
- Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc 2013;2:e000421. [PubMed]
- Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2013;62:300-5. [PubMed]
- Javaheri S. Sleep disorders in systolic heart failure: a prospective study of 100 male patients. The final report. Int J Cardiol 2006;106:21-8. [PubMed]
- Sin DD, Fitzgerald F, Parker JD, et al. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999;160:1101-6. [PubMed]
- MacDonald M, Fang J, Pittman SD, et al. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med 2008;4:38-42. [PubMed]
- Kaneko Y, Floras JS, Usui K, Plante J, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003;348:1233-41. [PubMed]
- Kasai T, Narui K, Dohi T, et al. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest 2008;133:690-6. [PubMed]
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34-41. [PubMed]
- Chaouat A, Weitzenblum E, Krieger J, et al. Pulmonary hemodynamics in the obstructive sleep apnea syndrome. Results in 220 consecutive patients. Chest 1996;109:380-6. [PubMed]
- Sanner BM, Doberauer C, Konermann M, et al. Pulmonary hypertension in patients with obstructive sleep apnea syndrome. Arch Intern Med 1997;157:2483-7. [PubMed]
- Bady E, Achkar A, Pascal S, et al. Pulmonary arterial hypertension in patients with sleep apnoea syndrome. Thorax 2000;55:934-9. [PubMed]
- Fletcher EC, Schaaf JW, Miller J, et al. Long-term cardiopulmonary sequelae in patients with sleep apnea and chronic lung disease. Am Rev Respir Dis 1987;135:525-33. [PubMed]
- Motta J, Guilleminault C, Schroeder JS, et al. Tracheostomy and hemodynamic changes in sleep-inducing apnea. Ann Intern Med 1978;89:454-8. [PubMed]
- Arias MA, García-Río F, Alonso-Fernández A, et al. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure: a randomized, controlled cross-over study. Eur Heart J 2006;27:1106-13. [PubMed]
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001;163:19-25. [PubMed]
- Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53. [PubMed]
- Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034-41. [PubMed]
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010;182:269-77. [PubMed]
- Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med 2010;6:131-7. [PubMed]
- Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med 2009;180:36-41. [PubMed]
- Bravata DM, Concato J, Fried T, et al. Continuous positive airway pressure: evaluation of a novel therapy for patients with acute ischemic stroke. Sleep 2011;34:1271-7. [PubMed]
- Ryan CM, Bayley M, Green R, et al. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke 2011;42:1062-7. [PubMed]
- Hsu CY, Vennelle M, Li HY, et al. Sleep-disordered breathing after stroke: a randomised controlled trial of continuous positive airway pressure. J Neurol Neurosurg Psychiatry 2006;77:1143-9. [PubMed]
- Parra O, Sánchez-Armengol A, Bonnin M, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J 2011;37:1128-36. [PubMed]
- Antonelli Incalzi R, Marra C, Salvigni BL, et al. Does cognitive dysfunction conform to a distinctive pattern in obstructive sleep apnea syndrome? J Sleep Res 2004;13:79-86. [PubMed]
- Beebe DW, Groesz L, Wells C, et al. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep 2003;26:298-307. [PubMed]
- Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest 2012;141:1601-10. [PubMed]
- Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology 2013;18:61-70. [PubMed]
- Peppard PE, Szklo-Coxe M, Hla KM, et al. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med 2006;166:1709-15. [PubMed]
- Pillar G, Lavie P. Psychiatric symptoms in sleep apnea syndrome: effects of gender and respiratory disturbance index. Chest 1998;114:697-703. [PubMed]
- Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: Contribution of daytime sleepiness to residual depressive symptoms. Sleep Med 2010;11:552-7. [PubMed]
- Wells RD, Freedland KE, Carney RM, et al. Adherence, reports of benefits, and depression among patients treated with continuous positive airway pressure. Psychosom Med 2007;69:449-54. [PubMed]
- Bardwell WA, Ancoli-Israel S, Dimsdale JE. Comparison of the effects of depressive symptoms and apnea severity on fatigue in patients with obstructive sleep apnea: a replication study. J Affect Disord 2007;97:181-6. [PubMed]
- Ho ML, Brass SD. Obstructive sleep apnea. Neurol Int 2011;3:e15. [PubMed]
- Vorona RD, Ware JC. Sleep disordered breathing and driving risk. Curr Opin Pulm Med 2002;8:506-10. [PubMed]
- George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001;56:508-12. [PubMed]
- Strohl KP, Brown DB, Collop N, et al. An official American Thoracic Society Clinical Practice Guideline: sleep apnea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am J Respir Crit Care Med 2013;187:1259-66. [PubMed]
- Togeiro SM, Carneiro G, Ribeiro Filho FF, et al. Consequences of obstructive sleep apnea on metabolic profile: a Population-Based Survey. Obesity (Silver Spring) 2013;21:847-51. [PubMed]
- Bonsignore MR, Borel AL, Machan E, et al. Sleep apnoea and metabolic dysfunction. Eur Respir Rev 2013;22:353-64. [PubMed]
- Newman AB, Nieto FJ, Guidry U, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol 2001;154:50-9. [PubMed]
- Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol (1985) 2005;99:1998-2007. [PubMed]
- Punjabi NM, Beamer BA. Alterations in Glucose Disposal in Sleep-disordered Breathing. Am J Respir Crit Care Med 2009;179:235-40. [PubMed]
- Reichmuth KJ, Austin D, Skatrud JB, et al. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med 2005;172:1590-5. [PubMed]
- Resnick HE, Redline S, Shahar E, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care 2003;26:702-9. [PubMed]
- Kent BD, Grote L, Ryan S, et al. Diabetes mellitus prevalence and control in sleep-disordered breathing: the European Sleep Apnea Cohort (ESADA) study. Chest 2014;146:982-90. [PubMed]
- Harsch IA, Schahin SP, Radespiel-Tröger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2004;169:156-62. [PubMed]
- Shpirer I, Rapoport MJ, Stav D, et al. Normal and elevated HbA1C levels correlate with severity of hypoxemia in patients with obstructive sleep apnea and decrease following CPAP treatment. Sleep Breath 2012;16:461-6. [PubMed]
- Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med 2011;365:2277-86. [PubMed]
- West SD, Nicoll DJ, Wallace TM, et al. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 2007;62:969-74. [PubMed]
- Weinstock TG, Wang X, Rueschman M, et al. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep 2012;35:617-625B. [PubMed]
- Luyster FS, Kip KE, Drumheller OJ, et al. Sleep apnea is related to the atherogenic phenotype, lipoprotein subclass B. J Clin Sleep Med 2012;8:155-61. [PubMed]
- Tan KC, Chow WS, Lam JC, et al. HDL dysfunction in obstructive sleep apnea. Atherosclerosis 2006;184:377-82. [PubMed]
- Mirrakhimov AE, Ali AM. Pathobiology of obstructive sleep apnea-related dyslipidemia: focus on the liver. ISRN Cardiol 2013;2013:687069.
- Savransky V, Jun J, Li J, et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res 2008;103:1173-80. [PubMed]
- Steiropoulos P, Tsara V, Nena E, et al. Effect of continuous positive airway pressure treatment on serum cardiovascular risk factors in patients with obstructive sleep apnea-hypopnea syndrome. Chest 2007;132:843-51. [PubMed]
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735-52. [PubMed]
- International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. Available online: http://www.idf.org/webdata/docs/MetS_def_update2006.pdf, accessed 11/6/2014.
- Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab 2000;85:1151-8. [PubMed]
- Bonsignore MR, McNicholas WT, Montserrat JM, et al. Adipose tissue in obesity and obstructive sleep apnoea. Eur Respir J 2012;39:746-67. [PubMed]
- Tanné F, Gagnadoux F, Chazouillères O, et al. Chronic liver injury during obstructive sleep apnea. Hepatology 2005;41:1290-6. [PubMed]
- Jouët P, Sabaté JM, Maillard D, et al. Relationship between obstructive sleep apnea and liver abnormalities in morbidly obese patients: a prospective study. Obes Surg 2007;17:478-85. [PubMed]
- Daltro C, Cotrim HP, Alves E, et al. Nonalcoholic fatty liver disease associated with obstructive sleep apnea: just a coincidence? Obes Surg 2010;20:1536-43. [PubMed]
- Chin K, Nakamura T, Takahashi K, et al. Effects of obstructive sleep apnea syndrome on serum aminotransferase levels in obese patients. Am J Med 2003;114:370-6. [PubMed]
- Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009;5:263-76. [PubMed]


