Sedation or general anesthesia for transcatheter aortic valve implantation (TAVI)
Background
Aortic valve stenosis is the most common valvular disease in elderly patients and a major cause of mortality and morbidity. At the beginning of 2014 more than 507 million inhabitants were living in 28 countries of the European Union. Of these, 8.7% were older than 75 years old (1). Untreated symptomatic severe aortic stenosis is associated with up to 50% mortality at 2 years (2) and its prevalence is reported to be between 1-7% in persons older than 75 years (3,4). Approximately 1,000,000 patients with clinical severe aortic stenosis are estimated to live currently in 19 European countries and a further 540,000 in the United States (3,5). While younger patients are more likely to undergo surgical aortic valve replacement (SAVR), patients of higher age and concomitant comorbidities are at high risk for SAVR (6).
Since the initial CE approval in 2007 transcatheter aortic valve implantation (TAVI) has become an established therapy for this patient-group. To date more than 100,000 TAVI procedures have been performed worldwide with a focus on Europe and especially Germany (5).
Growing experience, further development of the devices, expansion of the indication to “intermediate-risk” patients, and economic considerations (7,8) have led to an increasing interest and discussion about performing TAVI under sedation (TAVI-S) as opposed to TAVI under general anesthesia (TAVI-GA).
Trial data
Between 2008 and 2014, 13 non-randomised trials have reported data on a total of 6,718 patients of whom 3,227 underwent TAVI-S (Table 1). These studies will be addressed in chronologic order.
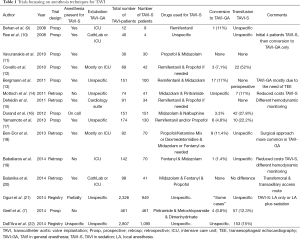
Full table
In 2008, Behan (9) et al., being among the first to address this topic, reported a small case series. After three initial TAVI-GA patients (of whom one died during the procedure), nine further TAVI-S patients were described. In favour of TAVI-S, Behan proposed a shortened procedure time and hospital stay. Furthermore, fewer complications and the avoidance of transferring the patient intubated to intensive care unit (ICU) were discussed.
In a similar period [2008], Ree et al. (10) described their experience with TAVI-S. After an initial four TAVI-S patients who required unplanned vascular surgery, their institutional policy subsequently favoured TAVI-GA (10).
Vavuranakis et al. (11) reported in 2010 successful valve implantation in 30 TAVI-S patients. The author proposed the feasibility of TAVI-S in two centers based on the absence of severe complications.
Covello [2010] (12) described a total of 69 patients (27 TAVI-GA, 42 TAVI-S) undergoing transfemoral or subclavian TAVI. TAVI-GA was primarily used during the initial learning phase and later in selected challenging patients with heart failure, obesity or restlessness. The authors did not present an analytic differentiation between transfemoral and subclavian access. A mean ventilation time of 8.5 hours was reported for TAVI-GA. Three TAVI-S patients (7%) required conversion to general anesthetic. Independently, vascular access complications and periprocedural bleeding were named as the most common adverse events. The authors concluded that TAVI-S and TAVI-GA were both suitable treatment strategies.
Bergmann (13) reported their experience, in 2011, with both anesthesia techniques in 151 patients. Patients were selected for TAVI-GA during the initial learning phase and later on in the presence of special comorbidities (pulmonary disease, cardiogenic shock) or the need for periprocedural transesophageal echocardiography (TEE). Vascular complications were given as the most common reason for conversion to TAVI-GA (17%). ICU length of stay, 30 days and 1 year mortality were described as comparable between both groups. According to the author, TAVI-S seemed to be feasible in the presence of an experienced anesthesiologist.
After an initial experience of 33 TAVI-GA patients, Motloch [2011] (14) compared these patients to 41 subsequent TAVI-S patients. An anesthesiologist was not present during TAVI-S. The interventionalist was solely responsible for dosage adjustment of anesthetics, analgesics and adrenergic support. Despite a significantly higher peak central aortic blood pressure reported in the TAVI-S group, 14% of these patients were in need of periprocedural adrenergic support. Reduced labor costs were further reported as a benefit of TAVI-S.
In 2011, Dehédin (15) compared 34 TAVI-S patients to initial 91 TAVI-GA patients. Procedure time, use of catecholamines, and volume expansion were described to be significantly lower in TAVI-S. According to the authors, general anesthesia was considered to be the main trigger for periprocedural hemodynamic instability. However, almost one in four TAVI-S patients was in need of catecholaminergic therapy. Hemodynamic monitoring was simplified in the TAVI-S group: the interventionalist initiated invasive blood pressure monitoring early during the procedure, after administration of sedative agents. A lower periprocedural volume expansion was demonstrated in TAVI-S by presenting stable creatinine values. Periprocedural complications were reported to be equal. According to the authors, a simplified monitoring, shorter procedure time and less hemodynamic instability were benefits of TAVI-S.
With 151 patients in sedation only, Durand (16) used a different approach in 2012. Again, the interventionalist was responsible for the surveillance of the patient and the conduction of sedation. An anesthesiologist was described to be “on call” only. Conversion to surgical therapy was needed in five cases (3%). Seven patients (5.5%) were in need of periprocedural vasopressor therapy. Hemodynamic stability, less need of vasopressor/inotropic therapy, fewer vascular access site complications and also shorter procedural duration were argued as benefit. Combined safety at 30-day was compared to existing literature in order to demonstrate the feasibility.
In 2013, Yamamoto (17) investigated 174 patients (44 TAVI-GA, 130 TAVI-S) undergoing transfemoral TAVI. Again TAVI-GA was used during the initial learning period. A sedation failure was reported in six patients (5%). Similar procedural outcome in procedural success, 30-day mortality, and 30-day combined safety was used to compare both procedures. Furthermore, earlier recovery, shorter procedure time, ICU and hospital stay were given as evidence of the feasibility and benefits of sedation.
Ninety-two patients undergoing transfemoral TAVI were analyzed in 2013 by Ben-Dor (18). Twenty-two of these were TAVI-GA in the initial learning period, followed by 70 TAVI-S patients. Hemodynamic monitoring was described to be equal in both groups. A conversion to TAVI-GA was needed in eight patients (11%). Hemodynamic compromise and respiratory failure were the most common non-procedure related cause for sedation failure. Surgical cut-down and procedural complications were more common in TAVI-GA patients. Considering these limitations, the authors proposed a shorter procedure time and ICU stay as benefits of sedation.
In 2014, Balanika (20) compared 57 TAVI-GA to 41 TAVI-S patients undergoing transfemoral or transaxillary TAVI. TAVI-GA patients were extubated either in the CathLab or on ICU, while all sedated patients were allowed to gain full consciousness in the CathLab. Mean time to extubation after the end of the procedure was 224 (±370) minutes. While the duration of the procedure was described to be comparable, significantly longer anesthesia duration was reported for the TAVI-GA group. No TAVI-S patients required conversion to TAVI-GA. Vasopressor therapy was needed in 18 (32%) TAVI-GA and 11 (26%) TAVI-S patients.
Seventy-two TAVI-GA procedures in a Hybrid-OR were compared to 70 TAVI-S patients in a CathLab by Babaliaros (19) in 2014. TAVI-GA patients received full hemodynamic monitoring (including pulmonary artery catheter), and were transferred to ICU for extubation. In contrast TAVI-S received a minimalistic approach without extended hemodynamic monitoring. Patients’ sedation was solely under the responsibility of the interventionalist. One of these patients needed to be converted to TAVI-GA. Procedure time, room time, and length of ICU stay were described as statistically significantly lower in the TAVI-S group. Stroke, bleeding complications, and new pacemaker implantation were reported as comparable. The authors pointed out especially the cost effectiveness of this minimalistic approach.
2014 data from the FRANCE 2 TAVI registry were analyzed by Oguri (21) to compare clinical outcome and safety. A total of 1,377 TAVI-GA patients were compared with 949 in the TAVI-S group. TAVI-S was defined as local anesthesia (LA) only or LA in addition to sedation. Conversion rate was not specified and reported as “some cases”. Procedural success, 30-day mortality, and the incidence of VARC defined complications were described as equal.
Greif (7) reported 461 patients of a German TAVI-S only program in 2014. As patient surveillance by an anesthesiologist was not considered to be necessary, the interventionalist was also responsible for the conduction of sedation. Life threatening hemorrhage occurred in 22 patients and major vascular complications were reported in 20 patients. Four patients died in the CathLab and a total of 21 patients had to be transported to the OR. The author justified their periprocedural strategy by comparing their data to those published by various authors.
Dall’Ara et al. (22) recently (2014) analyzed data from ten European countries contributing to the European Society of Cardiologist’s Transcatheter Valve Treatment (TCVT) Registry. The authors compared 1,712 TAVI-GA with 1,095 TAVI-S patients undergoing transfemoral TAVI. Baseline data revealed a higher Log-EuroSCORE, and more NYHA III-IV TAVI-S patients. Procedure and fluoroscopy time (mean: 23 vs. 34 min) were longer in TAVI-GA. TEE was rarely used in TAVI-S. A higher immediate procedural success rate and a lower rate of peri-procedural complications, such as requirement for permanent pacemaker implantation and cardiac tamponade, were shown in TAVI-GA. In contrast, TAVI-S patients had a shorter in-hospital time. Acute kidney injury (AKI) was more often diagnosed in TAVI-S, although a higher proportion of TAVI-GA patients received renal replacement therapy. In-hospital mortality was not independently associated with the type of anesthesia. One-year survival was also shown to be equal.
Discussion
Evidence guiding the decision of whether to perform TAVI under GA or conscious sedation is limited to non-randomized trials and registry data. Furthermore, the heterogeneous nature of these studies is an additional impediment to drawing any firm conclusions. Current evidence is limited by probable patient selection bias, methodological variability between studies and a lack of agreement regarding appropriate clinical end-points.
Despite the expansion towards “intermediate” or even “low-risk” patients, TAVI remains a procedure performed predominantly in aged patients with a high rate of comorbidities. Registries have reported a mean age of 81 years for these patients and, hence, pathophysiological changes in the elderly patient require careful consideration during procedures with the potential for serious hemodynamic disturbance, regardless of the mode of anesthetic utilized.
Sedation and general anesthesia
Sedation describes a state of reduced consciousness progressing via mild, moderate and deep sedation, to general anesthesia. The American Society of Anaesthesiologists (ASA) has published definitions of the different levels of sedation (23) and guidelines for the administration of sedation by non-anesthesiologists (24). Procedural sedation-related adverse events and hypoxia occur in up to 21% (25) of cases. Physicians without formal anesthetic training commonly perform conscious sedation during invasive procedures for relief of pain and anxiety symptoms. The guidelines stress the importance of appropriate patient selection, adequate monitoring during the procedure, suitable physician training for the level of sedation utilized and availability of medical staff with advanced life support and intubation skills. Furthermore, specialist anesthetic consultation is recommended for patients with risk factors for complications during sedation, including; advanced age, obesity, and patients with significant comorbidities.
As described above, the majority of TAVI patients have risk factors for complications during procedural sedation.
Hypotonia of the hypopharyngeal muscles (26) and an increased incidence of obstructive sleep apnea has been described in in up to 75% of elderly patients (27). All sedatives and opioid analgesics affect respiration to a certain degree and may also further reduce pharyngeal muscle tonus. Mild hypercapnia, induced by anesthesia, has shown to impair the coordination between swallowing and respiration and may therefore elevate the risk of aspiration (28). Furthermore, concentrations of remifentanil, as commonly used for monitored anesthesia care, have been associated with an increased risk of aspiration (29).
Literature describes an incidence of pulmonary-arterial hypertension (PAH) of up to 50% in TAVI patients (30). Sedation-related respiratory depression, hypercarbia, and acidosis may increase PAH and lead to right ventricular failure (31) potentially reducing the benefit of TAVI-S for such patients.
Periprocedural vasopressor therapy was consistently lower in the TAVI-S patients in these trials. Preexisting hypovolemia in combination with the vasodilatory effect of anesthetic agents may lead to hypotension in patients undergoing GA and thus explain the increased requirement for vasopressor therapy in this group. Nevertheless, vasopressors were used in up to 26% of TAVI-S (20). Preexisting hypovolemia or periprocedural bleeding may be causative, but as these data are not given, this remains speculative.
Complications occurring during TAVI-S may result in a need for unplanned intubation. The requirement for conversion to GA has been shown to be as high as 17% (13). Vascular complications requiring surgical intervention were given as the most common indication. Emergency or urgent induction of general anesthesia is often accompanied by hypotension. Green et al. revealed postintubation hemodynamic instability to occur in 11-44% of emergency in-hospital intubations (32). Chronic pulmonary obstructive disease, increased age and pre-intubation hemodynamic instability were associated with postintubation hemodynamic instability (32,33). As these three factors may frequently coexist in urgent TAVI conversion, the risk of hemodynamic instability in such patients is expected to be high. Consequently the presence of an experienced cardiac anesthesiologist would seem to be a necessity.
Time saving is frequently proposed as an advantage of a TAVI-S strategy, however, where the relevant information is provided in these studies, marked variability and complexity exists in the choice of hemodynamic monitoring utilized. As a result, time-consuming complex monitoring may limit the utility of procedure duration, or CathLab time, as a valid endpoint. Furthermore, the learning curve inherent to operators performing TAVI and the utilization of an arterial cut-down technique are likely to bias time end-point measurements in favour of TAVI-S. Procedure time was often not defined. As TAVI-GA patients were sometimes partially extubated in the ICU, time and place to gain full consciousness were different to TAVI-S patients. Therefore, the ICU time and the time to mobilize the patient inevitably had to be longer. If the patient is extubated on the table, the time to mobilization should normally depend on the process of the operation and sufficient postoperative analgesia (34). From the authors’ point of view, in TAVI patients undergoing femoral arterial access, effective and efficient vessel closure is probably the most important factor determining time to mobilization.
Postprocedural outcomes such as 30-day mortality (14,21), permanent pacemaker implantation (17,21), fluoroscopy time (19), and AKI (35) have also been used to compare TAVI-GA and TAVI-S. These endpoints reflect an overall procedural outcome. Within the first 48 hours after implantation cardiac causes are the predominant determinant of mortality. After day 15 non-cardiac causes, including sepsis, cancer and stroke appear to determine mortality (36).
With an incidence of 2-51%, permanent pacemaker implantation is common after TAVI. Device design, radial force exerted on the left ventricular outflow tract, and implantation technique may influence the requirement for subsequent permanent pacing. Preexisting conduction disturbances and periprocedural atrioventricular block have also been identified as risk factors (37).
The incidence of AKI is associated with decreased short- and long-term outcome after TAVI. AKI is reported in up to 28% of cases and is considered to be multifactorial (38,39). Inadequate kidney perfusion caused by hypotension during rapid ventricular pacing, debris and thromboembolism to the kidneys and contrast agent were argued as procedural causes. Although still under discussion, impaired preoperative renal function and dehydration has been shown to be associated with post-contrast AKI (40). Renal function is impaired in higher age. A decrease of renal blood flow, less cortical mass and sclerosis/remodeling of the glomeruli to nonfunctional tissue is additive to inadequate electrolyte and water intake. In conjunction with chronic diuretic therapy and preoperative fasting time, geriatric patients arrive at the operation room (OR) in a state of relative hypovolemia. Elhmidi recently described the incidence of AKI after TAVI between 8 and 57% (41). Blood transfusion, access route (transapical), preoperative creatinine clearance, hypertension, and perioperative bleeding were identified as risk factors (42). Unexpectedly the amount of contrast agent used was not associated with the incidence of AKI in this analysis. Nevertheless, only a small number of the described studies reported the amount of contrast used during the procedure (7,13,17,19). Hypotension may occur during the induction of general anesthesia. To date there is no evidence that general anesthesia itself is a risk factor for AKI. A recent analysis of 13,026 patients undergoing endovascular abdominal aortic aneurysm repair revealed that general anesthesia was not an independent risk factor for AKI (43).
Therefore, we consider standard endpoints such as the 30-day mortality rate, permanent pacemaker requirement, and AKI to be less useful for determining the appropriate anesthesiologic strategy in TAVI patients. Data about anesthesia-related peri- or very early postprocedural mortality and morbidity are not available.
When it comes to limited financial resources, the cost-effectiveness of medical procedures becomes increasingly important. Some authors have proposed TAVI-S as a more cost effective means of performing the procedure by avoiding the routine presence of an anesthetic team and thus reducing labor costs (7,14,16,19,42). This argument may be particularly appealing to physicians working in a fee-for-service setting (5). There is inadequate data available to determine whether performing TAVI-S, without start-to-finish anesthetic support, may be detrimental to patient care, however, current US (44), Australian (45), European (46), French (47) and German (48) TAVI-guidelines strongly recommend a “Heart Team” approach to patient care, with inclusion of a cardiac anesthesiologist. Performing TAVI procedures without an anesthesiologist in order to save time or money is not standard compliant and this fact should be taken into account when waiving the anesthesiologist for financial reasons.
Conclusions
General anesthesia and conscious sedation have both been used successfully to treat patients with severe aortic stenosis undergoing TAVI with similar reported short and long-term mortality outcomes. However, the anesthetic regimen itself remains just one part of a complex procedure for complex patients and a paucity of randomized data to guide practice has resulted in wide variation in the management of patients undergoing TAVI. Experienced high-volume TAVI centers continue to report very positive outcomes for patients treated with both sedation and general anesthesia. In the authors’ opinion, the significant incidence of complications and unplanned conversion to general anesthesia during TAVI-S mandates the start-to-finish presence of an experienced cardiac anesthetist in order to optimize patient outcomes. The decision to perform TAVI under conscious sedation or general anesthesia may ultimately be dictated by the experience of the Heart Team and local hospital policy, until good quality randomized data exists to inform practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- European Union—Eurostat. 1995-2014. Available online: , accessed on Nov 20, 2014.http://epp.eurostat.ec.europa.eu/portal/page/portal/statistics/search_database
- Chizner MA, Pearle DL, deLeon AC Jr. The natural history of aortic stenosis in adults. Am Heart J 1980;99:419-24. [PubMed]
- Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 2013;62:1002-12. [PubMed]
- Helske S, Kupari M, Lindstedt KA, et al. Aortic valve stenosis: an active atheroinflammatory process. Curr Opin Lipidol 2007;18:483-91. [PubMed]
- Osnabrugge RL, Kappetein AP, Reynolds MR, et al. Cost-effectiveness of transcatheter valvular interventions: economic challenges. EuroIntervention 2013;9 Suppl:S48-54. [PubMed]
- van Mieghem NM, Head SJ, van der Boon RM, et al. The SURTAVI model: proposal for a pragmatic risk stratification for patients with severe aortic stenosis. EuroIntervention 2012;8:258-66. [PubMed]
- Greif M, Lange P, Näbauer M, et al. Transcutaneous aortic valve replacement with the Edwards SAPIEN XT and Medtronic CoreValve prosthesis under fluoroscopic guidance and local anaesthesia only. Heart 2014;100:691-5. [PubMed]
- Covello RD, Landoni G, Zangrillo A. Anesthetic management of transcatheter aortic valve implantation. Curr Opin Anaesthesiol 2011;24:417-25. [PubMed]
- Behan M, Haworth P, Hutchinson N, et al. Percutaneous aortic valve implants under sedation: our initial experience. Catheter Cardiovasc Interv 2008;72:1012-5. [PubMed]
- Ree RM, Bowering JB, Schwarz SK. Case series: anesthesia for retrograde percutaneous aortic valve replacement--experience with the first 40 patients. Can J Anaesth 2008;55:761-8. [PubMed]
- Vavuranakis M, Voudris V, Vrachatis DA, et al. Transcatheter aortic valve implantation, patient selection process and procedure: two centres’ experience of the intervention without general anaesthesia. Hellenic J Cardiol 2010;51:492-500. [PubMed]
- Covello RD, Ruggeri L, Landoni G, et al. Transcatheter implantation of an aortic valve: anesthesiological management. Minerva Anestesiol 2010;76:100-8. [PubMed]
- Bergmann L, Kahlert P, Eggebrecht H, et al. Transfemoral aortic valve implantation under sedation and monitored anaesthetic care--a feasibility study. Anaesthesia 2011;66:977-82. [PubMed]
- Motloch LJ, Rottlaender D, Reda S, et al. Local versus general anesthesia for transfemoral aortic valve implantation. Clin Res Cardiol 2012;101:45-53. [PubMed]
- Dehédin B, Guinot PG, Ibrahim H, et al. Anesthesia and perioperative management of patients who undergo transfemoral transcatheter aortic valve implantation: an observational study of general versus local/regional anesthesia in 125 consecutive patients. J Cardiothorac Vasc Anesth 2011;25:1036-43. [PubMed]
- Durand E, Borz B, Godin M, et al. Transfemoral aortic valve replacement with the Edwards SAPIEN and Edwards SAPIEN XT prosthesis using exclusively local anesthesia and fluoroscopic guidance: feasibility and 30-day outcomes. JACC Cardiovasc Interv 2012;5:461-7. [PubMed]
- Yamamoto M, Meguro K, Mouillet G, et al. Effect of local anesthetic management with conscious sedation in patients undergoing transcatheter aortic valve implantation. Am J Cardiol 2013;111:94-9. [PubMed]
- Ben-Dor I, Looser PM, Maluenda G, et al. Transcatheter aortic valve replacement under monitored anesthesia care versus general anesthesia with intubation. Cardiovasc Revasc Med 2012;13:207-10. [PubMed]
- Babaliaros V, Devireddy C, Lerakis S, et al. Comparison of transfemoral transcatheter aortic valve replacement performed in the catheterization laboratory (minimalist approach) versus hybrid operating room (standard approach): outcomes and cost analysis. JACC Cardiovasc Interv 2014;7:898-904. [PubMed]
- Balanika M, Smyrli A, Samanidis G, et al. Anesthetic management of patients undergoing transcatheter aortic valve implantation. J Cardiothorac Vasc Anesth 2014;28:285-9. [PubMed]
- Oguri A, Yamamoto M, Mouillet G, et al. Clinical outcomes and safety of transfemoral aortic valve implantation under general versus local anesthesia: subanalysis of the French Aortic National CoreValve and Edwards 2 registry. Circ Cardiovasc Interv 2014;7:602-10. [PubMed]
- Dall’Ara G, Eltchaninoff H, Moat N, et al. Local and general anaesthesia do not influence outcome of transfemoral aortic valve implantation. Int J Cardiol 2014;177:448-54. [PubMed]
- American Society of Anesthesiologists. Continuum of depth of sedation: Definition of general anesthesia and levels of sedation/analgesia. Available online: http://www.asahq.org/~/media/legacy/for%20members/documents/standards%20guidelines%20stmts/continuum%20of%20depth%20of%20sedation.pdf
- American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology 2002;96:1004-17. [PubMed]
- Weaver CS, Hauter WE, Brizendine EJ, et al. Emergency department procedural sedation with propofol: is it safe? J Emerg Med 2007;33:355-61. [PubMed]
- Hoch CC, Reynolds CF 3rd, Monk TH, et al. Comparison of sleep-disordered breathing among healthy elderly in the seventh, eighth, and ninth decades of life. Sleep 1990;13:502-11. [PubMed]
- Ancoli-Israel S, Coy T. Are breathing disturbances in elderly equivalent to sleep apnea syndrome? Sleep 1994;17:77-83. [PubMed]
- D’Angelo OM, Diaz-Gil D, Nunn D, et al. Anesthesia and increased hypercarbic drive impair the coordination between breathing and swallowing. Anesthesiology 2014;121:1175-83. [PubMed]
- Savilampi J, Ahlstrand R, Magnuson A, et al. Aspiration induced by remifentanil: a double-blind, randomized, crossover study in healthy volunteers. Anesthesiology 2014;121:52-8. [PubMed]
- Ben-Dor I, Goldstein SA, Pichard AD, et al. Clinical profile, prognostic implication, and response to treatment of pulmonary hypertension in patients with severe aortic stenosis. Am J Cardiol 2011;107:1046-51. [PubMed]
- Minai OA, Yared JP, Kaw R, et al. Perioperative risk and management in patients with pulmonary hypertension. Chest 2013;144:329-40. [PubMed]
- Green R, Hutton B, Lorette J, et al. Incidence of postintubation hemodynamic instability associated with emergent intubations performed outside the operating room: a systematic review. CJEM 2014;16:69-79. [PubMed]
- Green RS, Edwards J, Sabri E, et al. Evaluation of the incidence, risk factors, and impact on patient outcomes of postintubation hemodynamic instability. CJEM 2012;14:74-82. [PubMed]
- Vizcaíno-Martínez L, Gómez-Ríos MÁ, López-Calviño B. General anesthesia plus ilioinguinal nerve block versus spinal anesthesia for ambulatory inguinal herniorrhapy. Saudi J Anaesth 2014;8:523-8. [PubMed]
- Barbanti M, Latib A, Sgroi C, et al. Acute kidney injury after transcatheter aortic valve implantation with self-expanding CoreValve prosthesis: results from a large multicentre Italian research project. EuroIntervention 2014;10:133-40. [PubMed]
- Van Mieghem NM, van der Boon RM, Nuis RJ, et al. Cause of death after transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2014;83:E277-82. [PubMed]
- Siontis GC, Jüni P, Pilgrim T, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J Am Coll Cardiol 2014;64:129-40. [PubMed]
- Barbash IM, Ben-Dor I, Dvir D, et al. Incidence and predictors of acute kidney injury after transcatheter aortic valve replacement. Am Heart J 2012;163:1031-6. [PubMed]
- Nicoara A, Patel UD, Phillips-Bute BG, et al. Mortality trends associated with acute renal failure requiring dialysis after CABG surgery in the United States. Blood Purif 2009;28:359-63. [PubMed]
- Meinel FG, De Cecco CN, Schoepf UJ, et al. Contrast-induced acute kidney injury: definition, epidemiology, and outcome. Biomed Res Int 2014;2014:859328.
- Elhmidi Y, Bleiziffer S, Deutsch MA, et al. Acute kidney injury after transcatheter aortic valve implantation: incidence, predictors and impact on mortality. Arch Cardiovasc Dis 2014;107:133-9. [PubMed]
- Bagur R, Webb JG, Nietlispach F, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J 2010;31:865-74. [PubMed]
- Kim M, Brady JE, Li G. Anesthetic technique and acute kidney injury in endovascular abdominal aortic aneurysm repair. J Cardiothorac Vasc Anesth 2014;28:572-8. [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1-e132. [PubMed]
- Walters DL, Webster M, Pasupati S, et al. Position statement for the operator and institutional requirements for a transcatheter aortic valve implantation (TAVI) program. Heart Lung Circ 2015;24:219-23. [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). G Ital Cardiol (Rome) 2013;14:167-214. [PubMed]
- Haute Autorité de Santé. Transcutaneous aortic valve implantation by the transfemoral or transapical route—Reassessment report. 2011. Accessed on Dec 18, 2014.
- Kuck KH, Eggebrecht H, Figulla HR, et al. Qualitätskriterien zur Durchführung der transvaskulären Aortenklappenimplantation (TAVI). Kardiologe 2015;9:11-26.


