Acute kidney injury after transcatheter aortic valve implantation
Introduction
Transcatheter aortic valve implantation (TAVI) has been established as an alternative procedure in cardiovascular medicine for the treatment of non-operable or high-risk patients with severe aortic stenosis. Over the last years, TAVI using mostly transfemoral (TF-AVI) and transapical (TA-AVI) access, has become an established procedure performed worldwide in highly qualified cardiac centers with satisfactory short- and mid-term results (1). Even though experience and techniques have constantly improved over the last years, peri- and postprocedural complications in high risk TAVI-collectives remain a major issue affecting outcome and survival (2). Taken together, preliminary data suggest that the minimally invasive transapical approach is a viable alternative for patients in who open heart surgery is not feasible or possesses unacceptable risk and may lead to a significant decrease in perioperative trauma and eventually to a decrease in perioperative risk. Despite these encouraging data this technique includes the need of fluoroscopy and angiography using contrast agent to aid positioning of the valve (3), which may lead to contrast-induced nephropathy (CIN) as one form or one etiology of acute kidney injury (AKI) which is associated with increased morbidity and mortality (4). Although previous studies established clinical and surgical characteristics associated with increased risk of AKI after cardiac surgery e.g., cardiopulmonary bypass (CPB) duration (5), severe hemodilution during CPB reflected by nadir hematocrit (6) and low oxygen delivery during CPB which are avoided by the lack of using CPB during TAVI, other potential factors like older age (5,7,8) diabetes mellitus, congestive heart failure still remain (5,7,8). Additionally, the systematic occurrence of short periods of extreme hypotension (rapid pacing for balloon valvuloplasty and valve deployment) and the manipulation of large catheters in the aorta of patients with a high prevalence of diffuse atherosclerosis with the risk of embolization are further potential risk factors for AKI after TAVI and may play an important role in terms of estimating the potential risk of this procedure influencing perioperative morbidity and mortality (9). This Review aims to present an overview over the current literature concerning AKI after TAVI with regard to the definition of AKI, the impact of AKI on mortality and potential risk factors for renal impairment after TAVI.
Definition of AKI after TAVI
AKI is defined as the rapid loss of kidney function occurring over hours or days and resulting in the dysregulation of volume and electrolyte homeostasis and in the accumulation of metabolic waste products (10). The definition of AKI in published studies dealing with the phenomenon of AKI after TAVI varies widely and lacks standardization. With regard to current definitions, the Acute Dialysis Quality Initiative (ADQI) group recommended the Risk, Injury, Failure, Loss, End-stage renal disease (RIFLE) criteria for diagnosis and classification of AKI. These definition, introduced in 2002, is based on either relative increases of serum creatinine or duration and severity of oliguria to classify AKI by defining the first stage (Risk) by a 1.5-fold increase in the serum creatinine, or a glomerular filtration rate (GFR) decrease by 25%, or urine output <0.5 mL/kg per hour for at least 6 hours (11). Due to the fact, that several studies suggested a relevant impact of even smaller changes in serum creatinine on patients outcome, the international consortium of renal and critical care societies [acute kidney injury network (AKIN)] recently proposed a modified classification. These modifications included the addition of an absolute increase in serum creatinine of ≥0.3 mg/dL and the specification that the decline in kidney function has to occur within 48 hours. Moreover, the AKIN proposed, that the term “acute renal failure” should be restricted to the severe state of complete organ dysfunction whereas the “Loss” and “End-stage” categories have been removed due to a lack of uniform indications and timing of renal replacement therapy (RRT) and variability of RRT availability in different countries (“AKIN criteria”) (12). With regard to the TAVI procedure, the Valve Academic Research Consortium (VARC) established an independent collaboration between Academic Research organizations and specialty societies to create consistent endpoint definitions and consensus recommendations for implementation in TAVI clinical research programs (13). In their first consensus report, the VARC proposed modified RIFLE criteria, implementing the 0.3 mg/dL increase in the “Risk” category for the definition of AKI after TAVI and additionally selected an outer bound of 72 hours after the index procedure for diagnosing AKI based on evidence that adverse outcomes were observed when the elevation occurred within 24 to 48 hours of the procedure and to ensure that the process was both acute and related to the procedure itself rather than as a consequence of post-procedure multi-organ system failure. Moreover, the VARC criteria did not include urine output criteria in their initial version since urine outputs may not be measured accurately or routinely in all cases. In 2012, the VARC published their updated endpoint definitions in the Valve Academic Research Consortium-2 (VARC-2) consensus document (2) now recommending the AKIN criteria including the definition of AKI according to urine output measures. Moreover, the timing for the diagnosis of AKI was extended from 72 hours to 7 days.
In conclusion, a broad variety of definitions was proposed since the establishment of the TAVI procedure resulting in a lack of standardization and therefore might be an additional explanation for different results concerning the incidence of AKI after TAVI. However, two aspects should be mentioned: the VARC-2 criteria implemented the most current definition of AKI, which has been also adopted by the majority of the nephrology community, including the KDIGO (Kidney Disease: Improving Global Outcomes) initiative (14) and should therefore serve as the standard definition for future studies (Table 1). Despite the advances in defining AKI, limitations remain, as all named definitions are predominantly based upon changes of serum creatinine levels. A creatinine elevation often occurs delayed in relation to the onset of AKI and serum creatinine levels are influenced by several other factors than kidney function, especially in elderly patients, in whom additionally alterations in volume status affect creatinine values. With regard to these shortcomings, several biomarkers are currently validated to allow an earlier diagnosis of AKI and to facilitate the differentiation between functional and structural kidney damage. The potential future implementation of novel biomarkers for the definition of AKI after TAVI might possibly affect diagnosis and treatment options of TAVI patients (10).
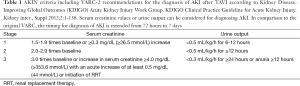
Full table
Pathophysiology of AKI after TAVI
As mentioned in the VARC-1 consensus document, the natural history of AKI in a variety of clinical settings is now well understood, including the recognition that even small decreases in kidney function can have a dramatic impact on the risk for subsequent mortality (13,15,16). However, some aspects concerning the pathophysiology of AKI are—even if not specific, but at least—typical for TAVI patients. Although the absence of CPB reflects a major difference in comparison to the classical cardiac surgery associated acute kidney injury (CSA-AKI), TAVI patients (at the moment) represent a special patient clientele with regard to the presence of comorbidities and often even concerning their age. At the age of 70, the kidneys have lost between 30% to 50% of their cortical glomeruli due to ischemic changes and a significant number of the remaining glomeruli manifest some degree of sclerosis, next to concomitant tubular and vascular changes leading to functional alterations including a reduction in renal blood flow of up to 50% from age 20 to age 80 (10,17). AKI in TAVI patients is most likely a combination of prerenal azotemia and direct nephrotoxic influences leading to renal ischemia and acute tubular necrosis (ATN). Causes of prerenal azotemia include (among others) hypovolemia, hemorrhage, impaired cardiac output or renal vasoconstriction caused by vasoconstrictive medication. The normal response of the kidney to prerenal conditions is to concentrate the urine and reabsorb sodium in order to maintain or increase intravascular volume and normalize renal perfusion. Therapy to restore renal perfusion promptly improves renal function. However, prolonged or profound prerenal azotemia can result in ischemic damage leading to ischemic AKI, particularly in combination with the presence of exogenous toxic compounds (e.g., aminoglycosides, contrast media) (18). This ischemic condition is leading to tubular damage. Thus, AKI after TAVI can be considered as the common final path resulting from prerenal azotemia due to pre-, intra- and postoperative factors and additional nephrotoxic influences resulting in ATN. A detailed review of the pathophysiology of AKI is outside the scope of this report and the reader is referred to a published review for further information (18). In summary, several processes lead to AKI after TAVI and the awareness of these factors at every particular timepoint before, during and after the TAVI procedure are of utmost importance to reduce the danger of postprocedural AKI.
Incidence and impact on mortality of AKI after TAVI
AKI is a frequent complication after TAVI being reported in ranges from 8.3% to 58% (9,19-24). Differing results might partially be explained by the use of different definitions of AKI. Moreover, the differences of the analyzed patient cohorts with regard to general comorbidities, access route (transfemoral, transapical or others), amount of contrast dye and especially known risk actors for the occurrence of AKI may account for the broad range of reported incidences. Currently, there is only one study focusing on AKI after TAVI using the AKIN criteria with a detailed observational period of 72 hours and a follow-up of renal function until postoperative day 7 (in line with the updated VARC II criteria). Sinning et al. (22) present in their sophisticated analysis the results of 77 patients who underwent TAVI and observed AKI in 26% of patients. The occurrence of AKI after TAVI was strongly associated with 30-day, 6-month and 1-year mortality. Konigstein et al. (25) analyzed the usefulness of the updated VARC-2 criteria with regard to AKI after TAVI and observed an incidence of 16.7% out of 251 patients without any patient suffering from stage III AKI. The major drawback of this study is the observational period. The authors did only focus on the first 72 hours after the procedure, although the updated VARC-2 criteria require, that the timing for the diagnosis of AKI should be extended from 72 hours to 7 days (VARC-II) (2). The only multicenter study published by Nuis et al. (19) used the VARC I criteria (modified RIFLE classification; period under consideration: 72 hours) to define postoperative AKI. The authors analyzed the outcome of 995 patients and report an incidence of AKI of 20.7% with a majority of patients presenting stage I (15%), whereas 6% of patients presented stage II or III. Multivariable analysis revealed a 3.15-fold higher mortality risk for patients with postoperative AKI without analyzing the impact of different stages of AKI on mortality. Saia et al. (24) used identical criteria for the definition of AKI (VARC 1), but report a significantly higher incidence of AKI with 41%. In line with Nuis et al. (19), the majority of patients developed stage I (78.6%), 9.5% stage II and 11.9% stage III AKI. The higher frequency of AKI might be explained by the high incidence of preoperative present chronic kidney disease (CKD) (87.3%) in the analyzed cohort. AKI did not appear as a risk factor for 30-day mortality but post-procedural AKI stage III was an independent predictor of 1-year death showing a more than 8-fold increased mortality risk. Seiffert et al. (26) analyzed 326 patients with regard to the VARC 1 endpoint definitions and observed an incidence of 29.5% AKI. Whereas stage I AKI was common and did not show significant impact on 1-year all-cause mortality, AKI stage II (HR 2.52; P=0.013) and stage III (HR 6.80; P<0.001) showed to be independent significant predictors for diminished 1-year survival. The lowest incidence of AKI after TAVI report Bagur and colleagues (9) in their analysis of 213 patients with an incidence of AKI of 11.7%. Despite these excellent results, it has to be mentioned, that the authors used the RIFLE classification for their analysis with a possible bias concerning AKI stage I. Moreover, the observational period was 48 hours in contrast to 72 hours in the studies mentioned above. However, even Bagur et al. identified AKI as independent predictor for in-hospital mortality. In the largest systematic review to asses predictors for periprocedural and midterm mortality after TAVI in high risk patients with symptomatic AS, analyzing 25 studies with more than 8,000 patients, AKI ≥ stage II (according to the VARC 1 definitions) was the strongest predictor for 30-day mortality, whereas stage III AKI was an important determinant for midterm mortality (27).
In conclusion, AKI is a common complication after TAVI and shows to be a strong predictor of short- and mid-term mortality. Only a few studies analyzed the severity of AKI in terms of the defined different stages (I-III), the majority of patients seem to develop “mild” AKI (stage I). Although there is evidence, that small decreases in kidney function can have dramatic impact on the risk for subsequent mortality (15,28), current data on AKI after TAVI do not support these findings with regard to AKI after TAVI. However, stage II and III seem to be independent relevant predictors for short- and mid-term mortality and are therefore also included in the “combined safety endpoint at 30 days” in the VARC endpoint definitions (2).
Risk factors for AKI after TAVI
Several studies identified risk factors for AKI after cardiac surgery and after percutaneous coronary intervention (PCI) (11,15). The TAVI procedure itself can currently be considered as a “hybrid procedure” using surgical and catheter-based techniques and valve implantation and can mostly be performed without the need for CPB, but the procedure requires the use of contrast agent. Thus, previous analyses focusing on either cardiac surgery associated-, or PCI associated AKI cannot be applied to the TAVI procedure. Moreover, the current patient clientele consists of multimorbide patients mostly presenting a multitude of identified risk factors for AKI. Besides, the TAVI procedure itself might influence the incidence of postoperative AKI due to technical aspects, as well as the different approaches (TF, TA, Tao, transsubclavian, etc.) might lead to a different outcome. And again, different definitions of AKI might result in statistical bias.
With regard to the current data concerning the outcome after TAVI, several studies identified predominant peripheral arterial occlusive disease (PAOD) as independent significant predictor for postoperative AKI (19,20,22,25). Next to the underlying generalized atherosclerosis including renal perfusion as important factor for the decrease of renal function, particulate atherosclerotic emboli generated during valvuloplasty, catheter passage through the aorta and deployment of the valve prosthesis might additionally be responsible for a postoperative decrease in eGFR.
Another commonly identified risk factor is red blood cell (RBC) transfusion during or after the procedure (9,19,25,29,30). Nuis et al. (19) identified the number of blood transfusions to be the strongest predictor of AKI with a distinct gradient of risk. Interestingly, the clinical triggers upon which one may decide to administer blood transfusions during TAVI were not associated with AKI, leading the authors to the conclusion, that clinicians should be more restrictive in their use of blood transfusions during TAVI and that the need of unequivocal criteria for the decision of blood transfusion is advocated. Although the intended effect of RBC transfusion is the improvement of organ function by increasing tissue oxygen delivery, transfused blood cells may contribute to organ injury because of changes that occur during storage. Due to several functional changes (e.g., loss of ability to generate nitric oxide, increased adhesiveness to vascular endothelium, release of procoagulant phospholipids and accumulation of proinflammatory molecules), stored RBCs may impair tissue oxygen delivery, promote a proinflammatory state and activate leucocytes (31-35).
With regard to proinflammatory cascades, several studies revealed a coherency between the development of a septic inflammatory response syndrome (SIRS) and AKI after TAVI. Aregger et al. (20) observed a pathological leucocyte count and fever without clinical focus in patients who developed AKI after TAVI. In line with this, Sinning et al. (22) found that 60% of the analyzed patients with AKI fulfilled the criteria of SIRS and showed significantly higher leucocyte counts and CRP levels 48 hours after TAVI. A possible relation between postoperative leucocyte count and AKI was also found in several other studies (19,36,37). The pathophysiological background is not completely understood yet, but may be triggered by RBC transfusion as well as by renal ischemia-reperfusion injury caused by the TAVI procedure caused by rapid pacing, intraoperative hypotension and the grade of aortic regurgitation after the procedure.
One other matter of debate is the impact of CKD on postoperative outcome and AKI. Even with regard to the endpoint “30-day and 1-year mortality”, existing data is contradictory (22,38-42). Noteworthy, Voigtländer et al. (41) mention in their analysis, that patients with severe impaired renal function were excluded from the PARTNER trial (43) and therefore the results of the PARTNER trial cannot necessarily be extended to patients with severe impaired renal function. However, it is well known from the Edwards SAPIEN Aortic Bioprosthesis European Outcome registry (44), that CKD is upon the strongest independent predictors of 1-year mortality. With regard to the occurrence of AKI after TAVI, Elhmidi et al. (21) and Seiffert et al. (26) identified a correlation between baseline renal function and the incidence of AKI, whereas Wessely et al. (39) did not find an association between CKD and AKI after TAVI. From a pathophysiological point of view, a possible impact of baseline renal function on the occurrence of AKI after TAVI is not surprising as at least concomitant pre-existing small-vessel disease leading to the risk for organ malperfusion may play an important role in this respect.
The impact of contrast agent on the occurrence of AKI after TAVI is still a matter of debate. Although most studies do not show a statistical impact of the dose of contrast agent on the incidence of AKI after TAVI (9,19,22,24,25,29), there is data suggesting that higher doses of contrast agent might have an impact on the occurrence of AKI after TAVI. Madershahian et al. (45) found a possible association between a higher incidence of CIN and 30-day mortality with regard to extensive use of contrast media during TA-AVI among high-risk patients with pre-existing renal impairment. Van Linden and colleagues (37) described in a series of 270 consecutive standard TA-AVI patients that postoperative AKI and RRT depend on the amount of intra-operative contrast agent. Regarding the amount of intra-operative contrast-agent the authors show, that patients with dialysis and acute renal failure received a higher dose of contrast-agent, which was statistically significant in the RRT group only. Beside thrombocytopenia and pathological leukocyte count in terms of SIRS, Van Linden et al. (37) identified a higher amount of contrast media as an independent risk factor for AKI. These findings are in line with the study of Yamamoto et al. (30), who analyzed 415 consecutive transfemorally treated TAVI patients and identified a relationship between contrast dose increment and high prevalence of AKI. Major drawbacks of most studies are the inability to determine, if the relation between increased contrast media and worse post-TAVI outcomes is associative or causative. In other words, did the contrast agent itself cause worse outcome or is increased contrast amount a consequence of a complicated procedure. The pathophysiological background in terms of a contrast induced acute vasoconstriction caused by the release of adenosine, endothelin and other vasoconstrictors leading to reduction in renal blood flow is clearly understood (46). However, the impact of these effects on renal function is still under debate. For a detailed review of the pathophysiology of contrast induced nephropathy, the reader is referred to a published review (47).
Anyhow, the multimorbid condition in the current era of TAVI and superimposed insults caused by the procedure (microembolic events, bleeding complications, etc.) might overrule (statistically observable) negative effects of contrast agent administration.
In this context, it is of interest that Ewe et al. (48) observed that patients treated via transapical TAVI (TA-AVI) received significantly less contrast agent in comparison to the transfemoral (TF-AVI) approach, suggesting that the TA-AVI may be a preferred approach in patients with concomitant impaired renal function. Unfortunately, the authors did not analyze postoperative outcome with regard to AKI. This observation is leading to the question whether there is a difference with regard to AKI in relation to the procedural approach (TA/TF). Seiffert et al. (26) found a higher incidence of stage I AKI in TA-AVI patients but a lower frequency of stage III in comparison to TF-AVI patients. In contrast, several studies identified the TA approach as risk factor for the occurrence of AKI (9,20,24,49). Aregger et al. (20) identified the transapical approach as significant independent predictor for AKI after TAVI in their series of 54 patients. These findings are supported by the analysis of Saia et al. (24) who report that the transapical approached emerged as the only significant predictor of AKI. The authors performed sophisticated multivariable analysis, excluded variables with a significant correlation with the TA-access (general anesthesia and RBC transfusion) and tested several statistical models in attempt to avoid overadjustment. However, the authors did not differentiate between the different stages of AKI impeding the interpretation of a potential clinical relevance of the observed renal injury. Several hypotheses are under discussion to explain these findings. The TA approach is mostly and more often performed under general anaesthesia with a higher amount of analgetic medication leading to hemodynamic depression and lower blood pressure which is compensated by the administration of vasoconstrictive drugs. These interventions cause impairments of general and renal perfusion, potentially leading to postoperative AKI. Besides, the duration of the weaning period after the intervention and consecutive differences in fluid management of the intensive care unit might additionally influence renal hemodynamics. Currently, there is no valid data focusing on these aspects. Moreover, as the “TF first strategy” is practiced at most centers, TA patients suffer present a higher risk profile in terms of comorbidities resulting in a higher susceptibility including a predisposition for renal injury. In line with this, patients selected to the transapical approach are typically selected for this approach because of advanced peripheral vascular atherosclerosis with highly calcified, tortuous, and small peripheral vessels. The presence of such advanced peripheral disease may be associated with a higher prevalence of calcium plaques also in the aorta and the aortic valve, which may embolize plaques to the kidneys during the procedure. Conversely, the transfemoral approach involves delivery of a large profile device in the abdominal aorta, which may cause embolization of aortic plaques into the renal arteries (49). However, next to the direct embolization, preexisting calcification might be more an indicator for “renal frailty” than a causative factor. This hypothesis is supported by the findings of van Rosendael et al. (50) who investigated the association between the atherosclerotic burden and plaque characteristics of the aortic valve and thoracic aorta and the incidence of AKI after TAVI. In this study, the burden of noncalcified atherosclerosis was independently associated with AKI (odds ratio, 1.03 per each 1% of increase in aortic segments with noncalcified atherosclerosis) after adjusting for baseline renal function, logistic EuroSCORE, and procedural access. In summary, current data suggest that patients treated via the transapical TAVI show a higher incidence of AKI in comparison to TF patients. However, current data allow no valid estimation with regard to the different hypotheses. Larger studies focusing on the incidence with regard to the different approaches might clarify the role of the access itself and the differences of the treated patient populations.
Preventive strategies
In order to reduce the risk of AKI after TAVI, preventive strategies, especially for patients with high risk for AKI deserve consideration. As mentioned in almost every study, the prevention of contrast induced tubular necrosis using N-acetylcystein or sodium bicarbonate and careful intravenous hydration based on the individual cardiac performance of each patient are practicable. Despite conflicting results with regard to the impact of contrast agent on AKI, the avoidance of unnecessary amount of contrast agent by performing hand-injections, dilution of media and gaining experience in echocardiographic guiding for valve positioning, as well as a precise preoperative planning should be mandatory with regard to the crucial influence of AKI on short- and mid-term mortality. Additionally, a more restrictive use of blood transfusions may improve renal outcome. Efforts to maximize the period of time between transcatheter aortic valve procedures and preoperative contrast based investigations might have beneficial effects. More elaborate methods like the implementation of Doppler-based renal resistance index might help to identify patients at risk (51). Further prophylactic strategies like the choice of the adequate contrast medium, withholding of nephrotoxic drugs, preventive dialysis or forced hydration may potentially reduce the risk of AKI, but cannot be generally recommended and need to be approved in further studies (10). To elaborate the optimal regime for each institution and, ideally, for each individual patient, close collaboration with the attending nephrologists is inevitable.
Comment
The pivotal role of AKI after TAVI on short- as well as mid-term mortality is beyond all questions. However, the distinct incidence and impact as well as the identification of risk factors are still matter of debate. The lack of standardization with regard to the definition of AKI in current studies complicates interpretation. Therefore, the updated VARC-2 criteria including the different stages of AKI should serve as the standard definition for AKI after TAVI in future studies including an observational period of 7 days after the procedure. Moreover, it would be favorable to analyze detailed procedural information such as duration of rapid pacing, predilatation of aortic annulus, intraoperative hypotension, perioperative inotropic support etc. to perform a sophisticated analysis with regard to possible risk factors to identify periprocedural changes that may contribute to renal insult. Beside the pure outcome analysis, a gain of experience and information concerning the side effects of TAVI is needed to fully understand the role of AKI after TAVI and to identify outcome predictors.
With regard to the presented data, it can be summarized that predominant PAOD and RBC transfusion during or after the procedure most obviously contribute to the development of AKI after TAVI. The impact of the applied contrast agent is still a matter of debate. However, with regard to the underlying pathophysiological mechanisms especially in older and fragile TAVI patients, attention should be drawn to every periprocedural aspect to protect these patients from renal damage. Prehydration, reduction of contrast administration, critical revision of the indication for blood transfusion, careful fluid management and avoidance of nephrotoxic drugs are important to optimize patients’ outcome. Having in mind, that severe AKI has been identified as the strongest predictor for 30-day mortality and as an important determinant for midterm mortality, even the pre- and postprocedural therapy must be taken into account and requires dedicated care in specialized centers.
In addition, further insight will improve patient selection and facilitate preoperative decision making concerning to the optimal approach (surgical aortic valve replacement or catheter-based treatment) and the favorable access route with regard to the TAVI procedure.
Acknowledgements
We thank the whole interdisciplinary TAVI team of our institution for their support, especially the head of the department of cardiology, Prof. Dr. S. Baldus and both team leaders, PD Dr. T. Rudolph (cardiology) and PD Dr. N. Madershahian (cardiac surgery).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vahanian A, Alfieri OR, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 2008;34:1-8. [PubMed]
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403-18. [PubMed]
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation 2006;114:591-6. [PubMed]
- Ranucci M, Ballotta A, Kunkl A, et al. Influence of the timing of cardiac catheterization and the amount of contrast media on acute renal failure after cardiac surgery. Am J Cardiol 2008;101:1112-8. [PubMed]
- Conlon PJ, Stafford-Smith M, White WD, et al. Acute renal failure following cardiac surgery. Nephrol Dial Transplant 1999;14:1158-62. [PubMed]
- Ranucci M, Pavesi M, Mazza E, et al. Risk factors for renal dysfunction after coronary surgery: the role of cardiopulmonary bypass technique. Perfusion 1994;9:319-26. [PubMed]
- Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 2004;44:1393-9. [PubMed]
- Mehta RH, Grab JD, O'Brien SM, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation 2006;114:2208-16. [PubMed]
- Bagur R, Webb JG, Nietlispach F, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J 2010;31:865-74. [PubMed]
- Abdel-Kader K, Palevsky PM. Acute kidney injury in the elderly. Clin Geriatr Med 2009;25:331-58. [PubMed]
- Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204-12. [PubMed]
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [PubMed]
- Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J 2011;32:205-17. [PubMed]
- Zeng X, McMahon GM, Brunelli SM, et al. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol 2014;9:12-20. [PubMed]
- Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol 2004;15:1597-605. [PubMed]
- Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis 2002;39:930-6. [PubMed]
- Hollenberg NK, Adams DF, Solomon HS, et al. Senescence and the renal vasculature in normal man. Circ Res 1974;34:309-16. [PubMed]
- Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2012;2:1303-53. [PubMed]
- Nuis RJ, Rodés-Cabau J, Sinning JM, et al. Blood transfusion and the risk of acute kidney injury after transcatheter aortic valve implantation. Circ Cardiovasc Interv 2012;5:680-8. [PubMed]
- Aregger F, Wenaweser P, Hellige GJ, et al. Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Nephrol Dial Transplant 2009;24:2175-9. [PubMed]
- Elhmidi Y, Bleiziffer S, Deutsch MA, et al. Acute kidney injury after transcatheter aortic valve implantation: incidence, predictors and impact on mortality. Arch Cardiovasc Dis 2014;107:133-9. [PubMed]
- Sinning JM, Ghanem A, Steinhäuser H, et al. Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation. JACC Cardiovasc Interv 2010;3:1141-9. [PubMed]
- Thomas M, Schymik G, Walther T, et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2010;122:62-9. [PubMed]
- Saia F, Ciuca C, Taglieri N, et al. Acute kidney injury following transcatheter aortic valve implantation: incidence, predictors and clinical outcome. Int J Cardiol 2013;168:1034-40. [PubMed]
- Konigstein M, Ben-Assa E, Abramowitz Y, et al. Usefulness of updated valve academic research consortium-2 criteria for acute kidney injury following transcatheter aortic valve implantation. Am J Cardiol 2013;112:1807-11. [PubMed]
- Seiffert M, Schnabel R, Conradi L, et al. Predictors and outcomes after transcatheter aortic valve implantation using different approaches according to the valve academic research consortium definitions. Catheter Cardiovasc Interv 2013;82:640-52. [PubMed]
- Giordana F, D'Ascenzo F, Nijhoff F, et al. Meta-analysis of predictors of all-cause mortality after transcatheter aortic valve implantation. Am J Cardiol 2014;114:1447-55. [PubMed]
- Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 2006;10:R73. [PubMed]
- Goebel N, Baumbach H, Ahad S, et al. Transcatheter aortic valve replacement: does kidney function affect outcome? Ann Thorac Surg 2013;96:507-12. [PubMed]
- Yamamoto M, Hayashida K, Mouillet G, et al. Renal function-based contrast dosing predicts acute kidney injury following transcatheter aortic valve implantation. JACC Cardiovasc Interv 2013;6:479-86. [PubMed]
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009;119:495-502. [PubMed]
- Almac E, Ince C. The impact of storage on red cell function in blood transfusion. Best Pract Res Clin Anaesthesiol 2007;21:195-208. [PubMed]
- Tinmouth A, Fergusson D, Yee IC, et al. Clinical consequences of red cell storage in the critically ill. Transfusion 2006;46:2014-27. [PubMed]
- Comporti M, Signorini C, Buonocore G, et al. Iron release, oxidative stress and erythrocyte ageing. Free Radic Biol Med 2002;32:568-76. [PubMed]
- Cardo LJ, Hmel P, Wilder D. Stored packed red blood cells contain a procoagulant phospholipid reducible by leukodepletion filters and washing. Transfus Apher Sci 2008;38:141-7. [PubMed]
- Nuis RJ, Van Mieghem NM, Tzikas A, et al. Frequency, determinants, and prognostic effects of acute kidney injury and red blood cell transfusion in patients undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2011;77:881-9. [PubMed]
- Van Linden A, Kempfert J, Rastan AJ, et al. Risk of acute kidney injury after minimally invasive transapical aortic valve implantation in 270 patients. Eur J Cardiothorac Surg 2011;39:835-42; discussion 842-3. [PubMed]
- Wendler O, Maccarthy P. Renal failure after transcatheter aortic valve implantation: do we know the full story? J Am Coll Cardiol 2013;62:878-80. [PubMed]
- Wessely M, Rau S, Lange P, et al. Chronic kidney disease is not associated with a higher risk for mortality or acute kidney injury in transcatheter aortic valve implantation. Nephrol Dial Transplant 2012;27:3502-8. [PubMed]
- Yamamoto M, Hayashida K, Mouillet G, et al. Prognostic value of chronic kidney disease after transcatheter aortic valve implantation. J Am Coll Cardiol 2013;62:869-77. [PubMed]
- Voigtländer L, Schewel J, Martin J, et al. Impact of kidney function on mortality after transcatheter valve implantation in patients with severe aortic valvular stenosis. Int J Cardiol 2015;178:275-81. [PubMed]
- Dumonteil N, van der Boon RM, Tchetche D, et al. Impact of preoperative chronic kidney disease on short- and long-term outcomes after transcatheter aortic valve implantation: a Pooled-RotterdAm-Milano-Toulouse In Collaboration Plus (PRAGMATIC-Plus) initiative substudy. Am Heart J 2013;165:752-60. [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [PubMed]
- Thomas M, Schymik G, Walther T, et al. One-year outcomes of cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2011;124:425-33. [PubMed]
- Madershahian N, Scherner M, Liakopoulos O, et al. Renal impairment and transapical aortic valve implantation: impact of contrast medium dose on kidney function and survival. Eur J Cardiothorac Surg 2012;41:1225-32. [PubMed]
- McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol 2008;51:1419-28. [PubMed]
- McCullough PA, Adam A, Becker CR, et al. Risk prediction of contrast-induced nephropathy. Am J Cardiol 2006;98:27K-36K. [PubMed]
- Ewe SH, Delgado V, Ng AC, et al. Outcomes after transcatheter aortic valve implantation: transfemoral versus transapical approach. Ann Thorac Surg 2011;92:1244-51. [PubMed]
- Barbash IM, Ben-Dor I, Dvir D, et al. Incidence and predictors of acute kidney injury after transcatheter aortic valve replacement. Am Heart J 2012;163:1031-6. [PubMed]
- van Rosendael PJ, Kamperidis V, van der Kley F, et al. Atherosclerosis burden of the aortic valve and aorta and risk of acute kidney injury after transcatheter aortic valve implantation. J Cardiovasc Comput Tomogr 2015;9:129-38. [PubMed]
- Sinning JM, Adenauer V, Scheer AC, et al. Doppler-based renal resistance index for the detection of acute kidney injury and the non-invasive evaluation of paravalvular aortic regurgitation after transcatheter aortic valve implantation. EuroIntervention 2014;9:1309-16. [PubMed]


