Midterm results of “treat and repair” for adults with non-restrictive ventricular septal defect and severe pulmonary hypertension
Introduction
A non-restrictive ventricular septal defect (VSD) can cause intracardiac left to right shunt. Without timely surgical correction, vasomotor dysfunction of endothelial cells and vascular remodeling will develop gradually in pulmonary artery, which leads to increased pulmonary vascular resistance (PVR) and pulmonary hypertension. Severe pulmonary hypertension causes bi-directional or even right-left shunt, manifested with cyanosis, hypoxemia, namely the Eisenmenger’s syndrome, which carries a poor prognosis (1,2). For patients with non-restrictive VSD with severe pulmonary hypertension at stage of near or to be Eisenmenger’s syndrome, traditional VSD repair carries high mortality and unsatisfactory prognosis for survivors. Qp/Qs <1.5 and Eisenmenger’s syndrome are considered to be the contraindication for surgery (3).
In the past few years, targeted drug therapy has been shown to decrease pulmonary circulation resistance in some patients with congenital heart diseases and even reverse right-left shunt to left-right shunt (4,5). Some scholars have attempted to perform defect repair surgery in drug treatment effective patients, namely “treat and repair” strategy (6,7). There are few reports about the midterm result of this strategy for adults with non-restrictive VSD and severe pulmonary hypertension at stage of near or to be Eisenmenger’s syndrome. The purpose of this study was to evaluate the midterm result of this surgical strategy in patients with this severe VSD whose PVR decreased obviously after targeted drug therapy.
Patients and the methods
Patients
The records of 41 adult patients with VSD and severe pulmonary hypertension were retrospectively reviewed. The patients underwent the VSD repair surgery between 2009 and 2013.
Inclusion criteria
- VSD whose diameter was over 1 cm without other intracardiac malformation;
- Severe pulmonary hypertension was diagnosed if preoperative mean pulmonary artery pressure (mPAP) was higher than 50 mmHg;
- Qp/Qs <1.5 in the initial right heart catheterization;
- Treated with targeted medical therapy before surgery for pulmonary hypertension, and underwent surgery ultimately;
- Patients were over the age of 18.
All patients received somatoscopy, chest radiography and blood gas analysis before and after medicine treatment. Color Doppler echocardiography and right heart catheterization were also routinely carried out.
Drug treatment
After the diagnosis was confirmed by initial right heart catheterization, all of the patients started taking sildenafil or bosentan, and for those without obvious effect after 3 months of treatment, inhaled iloprost. Patients were followed up in our outpatient clinic once every 3 months during treatment. If the patient has the following change during the follow-up, the right heart catheterization should be performed again:
- Systolic murmur along the left sternal border or louder existing murmur;
- Color Doppler echocardiography showed that bi-directional or right-left shunt turned to left-right shunt.
Surgical indication
According to the result of right heart catheterization reexamination, if Qp/Qs >1.5 and PVR/systemic vascular resistance (SVR) <2/3 (baseline or challenged with vasodilators), surgery could be performed. Right cardiac catheterization provocative tests adopted the method of inhaling 100% oxygen at early stage, which was replaced with 10 mg of iloprost aerosol inhalation at later stage.
Surgical approach
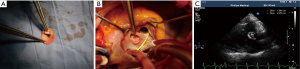
Surgeries were performed under general anesthesia, mild hypothermia and cardiopulmonary bypass (CPB). Pulmonary arterial pressure was monitored through Swan-Ganz catheter. After cardiac perfusion arrest, VSD was exposed through the right atrial or ventricular outflow tract, a polyester fiber heart patch was clipped to the size of defect, with a tap hole of 5 mm at center, for repairing VSD. Another polyester fiber heart patch was clipped into the size over the hole like a ping pong racket and seamed to the left ventricular side of the defect patch for opening or closing the tap hole along with bloodstream, namely “valve” (Figure 1A-C). When left ventricular pressure was higher than right ventricular pressure, “valve” closed the tap hole, otherwise valve opened and right-left shunt appeared. The left atrium drain was conventionally detained during operation, of which seven cases had the left atrium drain implanted through right atrium and atrial septal and out of the chest wall through the chest wall through intercostal space. And other 34 cases had deep venous catheter implanted into left atrium through atrial septum.
After heart resuscitation, hyperventilation was given, nitric oxide (NO) (10-20 ppm) or iloprost was inhaled, mPAP/mean aorta arterial pressure <0.8 was maintained and CPB was gradually removed. Protamine was injected from the ascending aorta to neutralize heparin.
Perioperative management
Early postoperative deep sedation was administered. Sildenafil 50-100 mg was poured through nasal feeding tube, NO or iloprost was inhaled. Cardiotonic agents and vascular contraction agents were pumped through left atrium drain. Since from the third day after surgery, the dosage of sedatives was gradually reduced for further revive. If pulmonary hypertension crisis appears, sedation should be continued. If the tracheal intubation was not extracted after a week, an emergency tracheotomy should be performed.
Pulmonary hypertension crisis treatment
The major clinical manifestations were that the pulmonary artery pressure was rapidly increased exceeding aorta arterial pressure; declined arterial oxygen saturation; hard-to-maintain systemic blood pressure; acidosis and oliguria in some serious cases. Treatment:
- Pure oxygen was inhaled with the increased alveolar ventilation;
- Deepening sedation, avoiding any stimulating operation;
- Increasing the dosage of NO or iloprost;
- If there was pulmonary infection; effective antibiotics should be adopted based on the sputum culture.
Follow-up
The patients were followed up in out—patient clinic once every 6 months after discharge with the last follow-up of September 2014. Somatoscopy, colour Doppler echocardiography and blood gas analysis were routinely carried out during follow-up. The mPAP was estimated by colour Doppler echocardiography. A total of 36 cases continued targeted medical therapy during follow-up, while three cases stopped for economic reasons.
Statistical methods
The data was analyzed by the software SPSS 17.0 statistically and shown in the form of mean ± standard deviation; t-test was used in the contrast of continuous data. Logistic regression model was adopted for risk factors analysis. P<0.05 means that the difference was statistically significant.
The study was approved by ethics committee of Renji Hospital affiliated to Shanghai Jiao Tong University School of Medicine and obtained patients’ informed consent.
Results
Patients’ characteristics
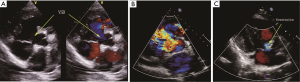
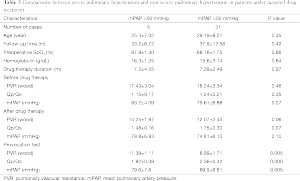
The basic information and the right heart catheterization result of patients were shown in Table 1. The age of 41 patients (19 males, 22 females) ranged from 19 to 60 years (average age, 28.15±8.14 years). The period of preoperative targeted medical therapy ranged from 3 to 15 months with an average of 7.61±3.00 months, including 18 cases of oral bosentan, 9 cases of oral sildenafil, 14 cases of oral sildenafil with inhaled iloprost. PVR was significantly decreased after treatment (17.08±3.97 vs. 12.76±2.73). A total of 35 cases were perimembranous VSD while six cases were subarterial VSD. The internal diameter was of 1.5-3.0 cm with the average of 2.20±0.37. Two cases combined muscular VSDs. Preoperative color Doppler echocardiography showed 24 cases of mild tricuspid valve regurgitation and 17 cases of mild to moderate tricuspid valve regurgitation (Figure 2A). In the initial right heart catheterization of this study, there were eight cases with Qp/Qs ≤1, namely Eisenmenger’s syndrome (8/41, 19%). After oral bosentan treatment for 12 to 15 months, reexamination found that Qp/Qs >1. In provocative tests with iloprost, PVR decreased significantly, PVR/SVR was 0.44±0.05 and Qp/Qs >1. The preoperative color Doppler echocardiography after oral bosentan treatment is shown in Figure 2B.

Full table
Operation procedure
Perioperative outcome is shown in Table 2. Two cases died during hospitalization for the overall mortality rate of 4.9%. All patients adopted valved patch repair, there were two cases combined muscular VSD implanting occlude device to obstruct muscular VSD for the site of the defect was close to ventricular apical and hardly to be exposed. The CPB time was 89.90±12.32 min, the aortic cross-clamp time was 52.66±11.57 min, the mechanical ventilation time was 68.66±33.36 min, and the ICU monitoring time was 5.37±1.91 d. Three cases were found pulmonary infection after operation, tracheotomy was done in four cases.

Full table
Six cases were found pulmonary hypertension crisis in perioperative period with the mPAP exceeding the mean aorta arterial pressure, color Doppler echocardiography showed the opened valve, the high right-left shunt through patch and the rapidly declined arterial oxygen saturation (Figure 2C). After treatment, four cases achieved remission, two cases died.
Follow-up results
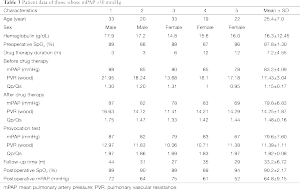
A total of 39 discharged patients were followed up for an average of 36.87±11.64 months. None of the patients died during follow-up. There were 36 cases continuing targeted medical therapy, mPAP was significantly reduced and SpO2 was significantly elevated, including 31 cases with mPAP <50 mmHg, of which the valve of tap hole was closed, for two of them, left-right shunt appeared, total pulmonary resistance was found to be considerably reduced by catheter, occlude device was implanted to obstruct the defect, and five other cases with mPAP >50 mmHg while continuing targeted medical therapy, namely severe pulmonary hypertension, the data is shown in Table 3.
Full table
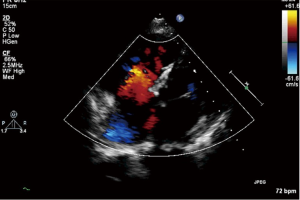
In eight cases of Eisenmenger’s syndrome after discharge in this study, there were three cases stopping targeted medical therapy for economic reason, of which the tap hole remained open during follow-up, the shunt was from right to left (Figure 3), the right ventricle was enlarged compared to preoperation, the tricuspid regurgitation was aggravated and SpO2 was declined, as compared to that in the early postoperative period, and five other cases adopting a combination of bosentan and iloprost during follow-up, including two cases of mPAP >50 mmHg, although it has been decreased than preoperative, as shown in Table 4.
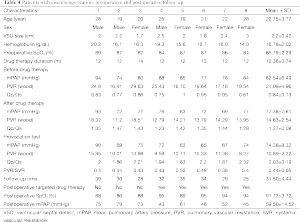
Full table
Risk factor after operation
Comparing preoperative variables of the five cases of severe pulmonary hypertension while continuing targeted medical therapy and the 31 cases with mPAP <50 mmHg, it was found that there were significant differences in Qp/Qs (challenged with vasodilators), PVR (challenged with vasodilators) and mPAP (challenged with vasodilators) between two groups. Further analysis with the logistic regression model showed that PVR (challenged with vasodilators) was an independent risk factor for postoperative severe pulmonary hypertension [P=0.02, Exp (B) =2.470], the data is shown in Table 5.
Full table
Discussion
According to statistics, about 50% of the non-restrictive VSD without timely repair which may cause severe pulmonary hypertension and develop into Eisenmenger’s syndrome eventually, which is rather difficult to treat. On the one hand, traditional repair surgery of VSD leads to rapid right heart function deterioration and high perioperative mortality because of the PVR approaching or briefly exceeding SVR (8-10). On the other hand, the right-left shunt and continuous hypoxemia of Eisenmenger’s syndrome cause reduced exercise tolerance, high hemoglobin, organ dysfunction and even cerebral infarction in serious cases (11,12). Meanwhile, the declined arterial oxygen saturation further causes the pneumoangiogram bed contraction and increased PVR, creating a vicious cycle. Some investigators attempt to perform stage operation on such patients that pulmonary artery banding is first carried out to reduce the pulmonary blood flow, if PVR decrease obviously during follow-up, then second-stage operation is undertaken to repair VSD (13). Other investigators reported a combination treatment of double lung transplant and VSD repair (14). The above methods only apply to a few cases for high risk and expense. In recent years, targeted agents against pulmonary hypertension, such as endostatin, prostaglandin analogues and phosphodiesterase type 5 (PDE5) inhibitor, etc., bring new future into patient survival status improvement. Endostatin—bosentan reduce the PVR and improve the exercise capacity of Eisenmenger’s syndrome in clinical randomized controlled trials (4). Prostaglandin analogues—iloprost also has been proved to be able to mitigate clinical symptoms alone or with prescription medications in clinical practice (15-17). However, there are many disputes on to what extent the PVR decreases after drug therapy can defect repair surgery be undertaken safely, how the postoperative long-term efficacy is, and what type of patients can benefit from it. All of 41 cases in this study accepted preoperative targeted medical therapy and the PVR declined significantly after treatment. The right-left shunt reversed meanwhile cyanosis and cardiac function improved in the eight cases of Eisenmenger’s syndrome. Right heart catheterization reexamination showed that Qp/Qs >1.5 in a resting or excited state, meanwhile PVR/SVR <2/3. Two cases died during the perioperative period for the overall mortality rate of 4.9%, which can be accepted.
Even under the effective targeted medical therapy, the pulmonary artery pressure of patients in this group was still too high with the preoperative mPAP of 75.95±6.61 mmHg; they may die because of pulmonary hypertension crisis, acute right heart failure and other factors during perioperative period. The two death cases in this study were also associated with pulmonary hypertension crisis. Novick and others designed a simple flapper valved patch (18,19) with one-way tap hole, when the right ventricular pressure is higher than the left ventricular pressure, blood can flow from right to left through the tap hole to reduce right ventricular volume load and guarantee the left ventricular cardiac output, wining for the treatment of time. In the early stage after operation, due to vascular contraction agents’ use, tissue edema, sputum suction, low oxygen, acidosis and other factors, hardly can transient elevation of pulmonary artery pressure even exceeding the aorta arterial pressure be complicatedly avoided (20). Therefore, all patients received flapper valved patch, once those happen during perioperative period, tap hole can temporarily relieve right ventricular pressure, and measures should be taken as soon as possible to reduce the pulmonary artery pressure at the same time. Usually by deepening calm, excess ventilation, inhaling NO or iloprost and other measures, pulmonary hypertension crisis could be reversed. During the follow-up, there were three cases of Eisenmenger’s syndrome stopping targeted medical therapy, of which pulmonary artery pressure rebounded and the valve remained open with bi-directional shunt, which protects the right ventricular function to some extent.
The pulmonary hypertension caused by non-restrictive VSD is mainly result from that a lot of bloodstreams from left heart flow into the pulmonary circulation for a long time, leading to pulmonary circulation overload. Rubin et al. found that if the shunt was removed, pulmonary arterial disorders could be reversed in animal experiments (21). We assume that pulmonary circulation resistance declines after drug therapy and pulmonary hypertension with left-right shunt and even Eisenmenger’s syndrome appear. Under postoperative continuing targeted medical therapy, pulmonary artery pressure can be maintained at a significantly lower level than that before operation, and the prognosis can be improved accordingly. In this study, there were 36 cases continuing targeted medical therapy, including 31 cases maintaining mPAP <50 mmHg with significantly improved transcutaneous oxygen saturation and cardiac function; and five other cases, of which although the pulmonary artery pressure declined than preoperative, mPAP >50 mmHg, namely severe pulmonary hypertension. Analyzing the data of five patients with preoperative right cardiac catheterization, found that Qp/Qs was greater than 1.5, but less than 2 under provocative tests, and the mPAP of four cases of them dropped limitedly. Comparing two groups, it was found that there were significant differences in Qp/Qs (challenged with vasodilators), PVR (challenged with vasodilators) and mPAP (challenged with vasodilators). Further analysis with the logistic regression model showed that PVR (challenged with vasodilators) was an independent risk factor for postoperative long-term severe pulmonary hypertension. Therefore, the result of provocative tests is an important aspect to be paying attention during the preoperative evaluation to screen the patients who can really benefit from it.
Whether Eisenmenger’s syndrome patients can benefit from “treat and repair” strategy? Studies have found that the pulmonary artery of one-third of the Eisenmenger’s syndrome patients still exists vasomotor function in a certain degree, and endostatin may reverse the pulmonary arterial disorders and improve pulmonary circulation by inhibiting cell proliferation (22,23). In this study, there were eight cases of Eisenmenger’s syndrome taking bosentan before surgery, three cases stopping targeted medical therapy, of which pulmonary artery pressure rebounded to pre-operation level, and other two cases continuing targeted medical therapy with decreased mPAP than preoperative, nevertheless it was still severe pulmonary hypertension. Therefore, surgery for such patients should be cautious; the provocative test result is an important aspect to be paying attention, meanwhile postoperative long-term targeted medical therapy should be taken.
Conclusions
For adults of non-restrictive VSD with severe pulmonary hypertension at stage of near or to be Eisenmenger’s syndrome, if the targeted medical therapy is effective and pulmonary circulation resistance reduces significantly, defect repair surgery should be considered with postoperative long-term targeted medical therapy for better midterm result.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Beghetti M, Galiè N. Eisenmenger syndrome a clinical perspective in a new therapeutic era of pulmonary arterial hypertension. J Am Coll Cardiol 2009;53:733-40. [PubMed]
- Adatia I, Kothari SS, Feinstein JA. Pulmonary hypertension associated with congenital heart disease: pulmonary vascular disease: the global perspective. Chest 2010;137:52S-61S. [PubMed]
- Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57. [PubMed]
- Galiè N, Beghetti M, Gatzoulis MA, et al. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation 2006;114:48-54. [PubMed]
- Dimopoulos K, Inuzuka R, Goletto S, et al. Improved survival among patients with Eisenmenger syndrome receiving advanced therapy for pulmonary arterial hypertension. Circulation 2010;121:20-5. [PubMed]
- Hu L, Tan LH, Ye J. Repair of ventricular septal defect with Eisenmenger syndrome after bosentan treatment. J Card Surg 2014;29:401-2. [PubMed]
- Liu YL, Hu SS, Shen XD, et al. Midterm results of arterial switch operation in older patients with severe pulmonary hypertension. Ann Thorac Surg 2010;90:848-55. [PubMed]
- Hoffman JI, Rudolph AM. The natural history of ventricular septal defects in infancy. Am J Cardiol 1965;16:634-53. [PubMed]
- Beghetti M. Pulmonary arterial hypertension related to congenital heart disease. Munich: Elsevier, 2006.
- Rao PS, Raju V, Narayana M. Flap valved closure of ventricular septal defects with increased pulmonary vascular resistance. Interact Cardiovasc Thorac Surg 2010;11:577-80. [PubMed]
- Diller GP, Dimopoulos K, Okonko D, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation 2005;112:828-35. [PubMed]
- Diller GP, Dimopoulos K, Broberg CS, et al. Presentation, survival prospects, and predictors of death in Eisenmenger syndrome: a combined retrospective and case-control study. Eur Heart J 2006;27:1737-42. [PubMed]
- Khan SA, Gelb BD, Nguyen KH. Evaluation of pulmonary artery banding in the setting of ventricular septal defects and severely elevated pulmonary vascular resistance. Congenit Heart Dis 2006;1:244-50. [PubMed]
- Inoue M, Minami M, Fukushima N, et al. Bilateral lung transplantation with closure of ventricular septal defect in a patient with Eisenmenger syndrome. Gen Thorac Cardiovasc Surg 2010;58:25-8; discussion 29. [PubMed]
- Olschewski H, Simonneau G, Galiè N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 2002;347:322-9. [PubMed]
- Olschewski H. Inhaled iloprost for the treatment of pulmonary hypertension. Eur Respir Rev 2009;18:29-34. [PubMed]
- McLaughlin VV, Oudiz RJ, Frost A, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med 2006;174:1257-63. [PubMed]
- Novick WM, Gurbuz AT, Watson DC, et al. Double patch closure of ventricular septal defect with increased pulmonary vascular resistance. Ann Thorac Surg 1998;66:1533-8. [PubMed]
- Novick WM, Sandoval N, Lazorhysynets VV, et al. Flap valve double patch closure of ventricular septal defects in children with increased pulmonary vascular resistance. Ann Thorac Surg 2005;79:21-8; discussion 21-8. [PubMed]
- Hopkins RA, Bull C, Haworth SG, et al. Pulmonary hypertensive crises following surgery for congenital heart defects in young children. Eur J Cardiothorac Surg 1991;5:628-34. [PubMed]
- Rubin LJ, Badesch DB. Evaluation and management of the patient with pulmonary arterial hypertension. Ann Intern Med 2005;143:282-92. [PubMed]
- Heath D, Helmholz HF Jr, Burchell HB, et al. Graded pulmonary vascular changes and hemodynamic findings in cases of atrial and ventricular septal defect and patent ductus arteriosus. Circulation 1958;18:1155-66. [PubMed]
- Marie Valente A, Rhodes JF. Current indications and contraindications for transcatheter atrial septal defect and patent foramen ovale device closure. Am Heart J 2007;153:81-4. [PubMed]