Predictors of dynamic hyperinflation during the 6-minute walk test in stable chronic obstructive pulmonary disease patients
Introduction
Exercise intolerance is a cardinal complaint of patients with chronic obstructive pulmonary disease (COPD) (1). Although the underlying mechanism of exercise intolerance is complex and multifactorial, dynamic hyperinflation (DH) remains a major contributor to exercise limitation in COPD (2). The relationship between DH and exercise capacity has been documented using cardiopulmonary exercise test (CPET) (3-5). In the clinical setting, CPET is often impractical because it requires specialized equipment, trained technicians, and physician supervision. Recent studies demonstrated that the 6-minute walk test (6MWT) provide a simple, safe, and well-tolerated method to detect DH in patients with COPD (6,7). However, the physiological factors responsible for DH development during the 6MWT have not been adequately evaluated. In addition, the effects of DH on ventilatory response to the 6MWT in COPD patients remain largely unknown. Finally, it is unclear whether the contribution to functional exercise capacity from resting lung function, demographic characteristics, exertional dyspnea and DH differs in COPD according to the DH status.
The aims of this study were: (I) to elucidate the physiological factors responsible for DH development during the 6MWT; (II) to compare physiological response to the 6MWT in hyperinflators and non-hyperinflators with COPD; and (III) to delineate the predictors of functional exercise capacity in hyperinflators and non-hyperinflators.
Methods
Participants
This prospective cross-sectional study was conducted between March 2013 and July 2014. Consecutive subjects with mild to very severe COPD from a pulmonary clinic at the Sun Yat-sen Memorial Hospital, Guangzhou, China were eligible for enrollment. COPD was diagnosed based on clinical manifestations, self-reported smoking history, and presence of persistent airflow limitation. Persistent flow limitation was defined as a post-bronchodilator forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) ratio <70% (8). The COPD severity was determined according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines.
The inclusion criteria were: (I) a history of COPD; (II) aged over 40 years; (III) pulse oximetric saturation (SpO2) at rest >90% on ambient air; and (IV) no change in medication dosage or symptoms during the preceding 4 weeks.
The exclusion criteria were: (I) serious cardiovascular diseases (including uncontrolled hypertension, unstable angina pectoris, recent myocardial infarction, or congestive heart failure); (II) a diagnosis of obstructive sleep apnea (OSA) by polysomnography (PSG) before enrollment; (III) evidence of malignancy or severe renal or hepatic dysfunction; and (IV) lower limb disability. Figure 1 summarizes the participant eligibility, exclusion, and enrollment process.
The study protocol was approved by the Ethics Committee, Sun Yat-sen Memorial Hospital, Guangzhou, China. All participants gave written informed consent. The study was registered at the Chinese Clinical Trial Registry (Registration Number: ChiCTR-TNRC-15006234, website: http://www.chictr.org/en/).
Group allocation
The study was performed at the Rehabilitation Laboratory of Guangdong Provincial Hospital, Guangzhou, China. Subjects were assigned to two groups based on occurrence of DH, defined as a decline in inspiratory capacity (IC) from baseline greater than zero (ΔIC >0.0 L) after 6MWT (9,10). Patients with a ΔIC >0.0 L in this study were diagnosed with DH (hyperinflators), and those with a ΔIC ≤0.0 L were considered DH negative (non-hyperinflators).
Measurements
Prior to examination, subjects were instructed to avoid strenuous exercise for 24 h. Demographic and clinical data were collected, followed successively by the pulmonary function test, 6MWT, assessment of quality of life and dyspnea. All measurements were obtained by a well-trained technician blinded to the study protocol.
Demographic and clinical data
The age, gender, smoking status, smoking history, body mass index (BMI), comorbidities, medication history, and use of oxygen therapy were recorded in each subject.
Pulmonary function tests
Standard forced expiratory spirometry was performed according to the American Thoracic Society guidelines using an electronic spirometer (CHEST GRAPH HI-101, Tokyo, Japan) before and after administering 400 µg salbutamol sulfate aerosol (Ventolin, GlaxoSmithKline, Shanghai, China) (11). The normal predicted values were determined according to the equations proposed by the European Coal and Steel Community with certain correction coefficients (12). Maximal voluntary ventilation (MVV) was estimated by multiplying FEV1 by 35.
Inspiratory capacity (IC) maneuvers
The IC was measured with the patient seated after fully explaining the procedure to each subject (13). Satisfactory and consistent technique for the IC maneuvers was established with the subject at rest. After four to six consistent normal breaths, the subject was instructed to inspire the total lung capacity (TLC) and then return to normal breathing. The two largest IC measurements of at least three acceptable trials were required to agree within 5%. The better of two reproducible maneuvers was recorded for analysis.
6-minute walk test (6MWT)
The 6MWT followed the recommendations from American Thoracic Society guidelines (14). Patients were given standardized instructions to walk as fast as possible for 6 min. IC was measured at rest and immediately after walking (within 60 s). The heart rate, breathing pattern, SpO2, and the modified Borg dyspnea scale (15) and leg fatigue ratings were recorded before and after walking. We measured lung volume by plethysmography in five subjects before and after the 6MWT. The results showed no significant changes in TLC, which indicated that TLC did not change during walking test. Assuming that TLC does not change during exercise, changes in the IC are used to indirectly reflect the level of DH (16,17).
Dyspnea and quality of life assessment
The degree of dyspnea was assessed using the Modified Medical Research Council (MMRC) dyspnea scale (18). The health-related quality of life (HRQL) was evaluated using the St George’s Respiratory Questionnaire (SGRQ) (19).
Statistical analysis
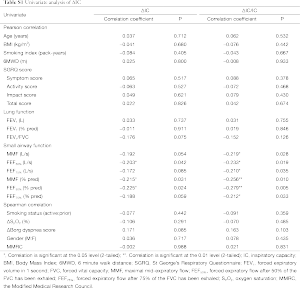
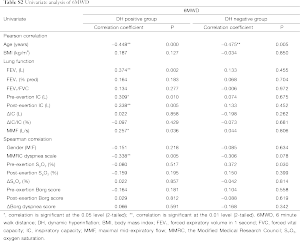
Data were expressed as the mean ± standard deviation. Statistical analyses were conducted using the Statistical Package for Social Sciences version 18.0. Chi-square analysis was applied to test discrete variables between the both groups. The independent samples t-test or Mann-Whitney U-test was used to compare continuous variables between the two groups. Pearson or spearman correlation coefficients were calculated for relationship between the ΔIC/IC or the 6-minute walk distance (6MWD) and other variables. Stepwise linear regression was performed to identify the factors independently associated with ΔIC/IC or the 6MWD. Variables were selected for use with the stepwise regression model based on the results of univariate analysis (Tables S1,S2 in the supplementary file). A P<0.05 was considered statistically significant.
Full table
Full table
Results
Subject characteristics
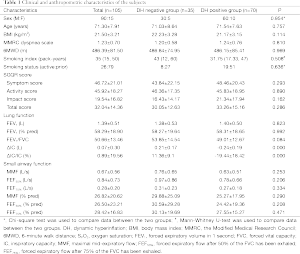
Table 1 displays the population characteristics. A total of 105 subjects with stable COPD were included in this study. The numbers of patients diagnosed with GOLD stages I, II, III, and IV were 16 (15.2%), 50 (47.6%), 31 (29.5.1%), and 8 (7.6%), respectively. The mean age was 71.34±7.95 years, and men comprised 85.71% of the sample. DH was present in 70 subjects (66.67%), with 10 subjects (10/16) at GOLD stage I, 34 subjects (34/50) at GOLD stage II, 20 subjects (20/31) at GOLD stage III, and 6 subjects (6/8) at GOLD stage IV. Although there were decreasing trends of small airway function and FEV1/FVC in hyperinflators, no significant differences were observed between the two groups.
Full table
Multiple regression analysis of DH
The univariate analysis of ΔIC/IC and ΔIC is shown in Table S1. Among the variables, only the maximal mid-expiratory flow (MMF) (% pred) (r=−0.256, P=0.010), the forced expiratory flow after exhaling 50% of the FVC (FEF50%) (% pred) (r=−0.279, P=0.005) and forced expiratory flow after exhaling 75% of the FVC (FEF75%) (% pred) (r=−0.212, P=0.033) were negatively correlated with the ΔIC/IC. No significant correlation was observed between FEV1 and ΔIC/IC (r=0.031, P=0.755). On multivariate regression analysis, the FEF50% was the only predictor of ΔIC/IC (Table 2). For the ΔIC/IC, the FEF50% uniquely contributed 27.9% of the variance.

Full table
The effects of DH on response to the 6MWT
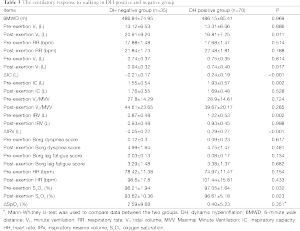
The response to the 6WMT in both groups is summarized in Table 3. There were no significant differences in the 6WMD between the two groups. IC decreased by 0.24±0.19 L at the end of walking in hyperinflators. The non-hyperinflators had a higher post-walking VT (t=2.419, P=0.017) and post-walking VE (t=2.599, P=0.011) than the hyperinflators did. No obvious difference was observed in the post-walking breathing frequency, Borg dyspnea scale, heart rate, and ΔSPO2 between the two groups. After the 6MWT, the decrease of inspiratory reserve volume (IRV) in hyperinflators was significant higher than those of non-hyperinflators (t=-6.226, P<0.001). In addition, the post-walking VT was significantly and positively correlated with the resting IC (r=0.293, P=0.004) and post-walking IC (r=0.456, P<0.001).
Full table
Multiple regression analysis of 6MWD
The univariate analysis of the 6MWD is shown in Table S2. In hyperinflators, age and resting IC were independent predictors of the 6MWD. These two factors contributed 60.1% of the variance for the dependent variable. In non-hyperinflators, age and MMRC dyspnea scale were the predictors of the 6MWD. These factors contributed 86.7% of the variance for the dependent variable (Table 2).
Discussion
There were several significant findings in this study. First, DH was common in the subjects with COPD and was easily detected using IC maneuvers during the 6MWT. Second, FEF50% was the only predictor of DH during the 6MWT in subjects with COPD. Third, the ventilatory response to the 6MWT differed between hyperinflators and non-hyperinflators. Finally, resting hyperinflation was an important predictor of functional exercise capacity in hyperinflators.
We found that 66.67% of subjects with COPD developed DH during the 6MWT. It indicated that DH was considerably common during the 6MWT for subjects with COPD. We did not observe any adverse events related to the IC maneuvers during the 6MWT, which suggests that performing IC maneuvers during the 6MWT is a simple, safe, and well-tolerated method of detecting DH. In this study, there was no significant difference in the airflow obstruction between hyperinflators and non-hyperinflators. In addition, no obvious correlation was observed between airflow obstruction and the severity of DH. These findings imply that airflow obstruction is a poor predictor of the DH, echoing previous findings by Hannink (20).
Many factors are reportedly associated with the occurrence of DH such as age (21), obesity (22), exertional desaturation (23), smoking history (24), and systemic inflammation, etc. However, few studies have focused on the effect of small airway function on DH. Stewart (25) reported that small airway inflammation and structural damage preceded a marked decrease of airflow obstruction, leading to air-trapping even in mild COPD patients. Interestingly, we also observed DH in subjects with mild COPD. Hence, we speculate that small airway obstruction may be related to DH. In the current study, there was a weak but significantly negative relationship between the small airway function and the extent of DH. Moreover, FEF50% was the only predictor of the DH severity. Our results suggest that small airway function may be partly responsible for the development of DH in patients with COPD. Further studies are warranted to demonstrate whether interventions to improve small airway function can reduce DH in patients with COPD. We did not observe the relationship between DH and age, BMI, exertional desaturation and smoking index. Zafar et al. (23) reported that DH strongly correlated with exertional desaturation during the 6MWT and could be a reason for this desaturation. Our result did not agree with those of Zafar et al. (23).The inconsistency might be underlain by the exclusion of the subjects with resting SpO2 less than 90% in our study. The resting SpO2 of our subjects was significantly higher than those of Zafar et al.
Patients with COPD experience increased ventilatory demand during exercise. O’Donnell (26) reported that during a high-intensity exercise test, the occurrence of DH constrains increases in tidal volume (VT). When close to minimal IRV, further increase of ventilatory demands is satiated through an increase in breathing frequency. However, the shortened expiratory time further exacerbates DH, creating a vicious circle. Our results showed that the post-walking VT was significantly and positively correlated with the resting IC and post-walking IC, which is consistent with previous findings (26). In addition, we observed a remarkable increase in the post-walking VT in non-hyperinflators instead of hyperinflators. There was an obvious increase in breathing frequency in both two groups. It indicated that the ventilatory response to the walking test differed between the two groups. In hyperinflators, the increased ventilatory demand was met primarily by the increase of breathing frequency. However, in non-hyperinflators, the increased ventilatory demand was satisfied by increasing both the breathing frequency and VT. Guenette (27) showed that IRV provided a better index of ventilation than hyperinflation during a high-intensity exercise test. However, we did not observe such relationships. Noticeably, we observed an obvious decrease of IRV at the end of walking in hyperinflators. In contrast, there was no obvious change of IRV in non-hyperinflators. This implied that hyperinflators was prone to appear VT constraint than non-hyperinflators during the 6MWD. O’Donnell (2) demonstrated that the ΔIC was significantly associated with exercise endurance under controlled laboratory conditions in COPD. In this study, although no obvious correlation was observed between the ΔIC and the 6WMD, both the resting IC and post-walking IC were positively correlated with the 6WMD. This suggests an indirect link between DH and exercise performance. We speculate that the inconsistency between our and previous findings is due to the different modes of exercise. The subjects didn’t reach their maximal exercise capacity and ventilatory response during the 6WMT.
The 6MWT is commonly used to assess functional exercise capacity in patients with COPD. In this study, there was no significant difference in the 6MWD between hyperinflators and non-hyperinflators. However, the regression equation for 6MWD is different between the two groups. Age and resting hyperinflation were the predictors of functional exercise capacity in hyperinflators, while age and MMRC dyspnea scale was the predictors of functional exercise capacity in non-hyperinflators. Our results are consistent with the findings of Callens (6). Potentially, interventions improving resting hyperinflation may improve the exercise capacity in hyperinflators, while interventions to alleviate dyspnea may improve exercise capacity in non-hyperinflators. Additional studies are needed to confirm the hypothesis.
This study has some limitations. First, we did not measure the TLC in all patients; therefore, it is unknown whether the TLC was unchanged during the walking test. Despite this, we measured TLC with plethysmography in five subjects before and after the 6MWT. The results showed no significant changes in TLC. Hence, we supposed that TLC did not change after the 6MWT. Second, the decrease in the IC during exercise was quickly resolved (6). Hence, IC maneuvers after the walking test may actually underestimate the true severity of DH, though in our study, post-walking IC was measured within 60 s after completing the walking test. Third, there was only a weak correlation between small airway function and the level of DH. Nevertheless, the relationship was of significantly statistical significance. Additional studies are required to validate our findings, using more accurate methods to detect small airway function, such as late-phase sputum (28), peripheral airway resistance (29), alveolar nitric oxide concentrations (30), and HRCT (31). Finally, other factors known to be associated with impaired exercise capacity in patients with COPD, such as anxiety, depression, skeletal muscle function (32), and cardiovascular abnormalities (33), were not assessed.
In conclusion, DH was common in patients with COPD and easily detected by performing IC maneuvers during the 6MWT. The FEF50% was the only predictor of DH during the 6MWT, and the ventilatory response to the 6MWT differed between hyperinflators and non-hyperinflators. Resting hyperinflation is an important predictor of functional exercise capacity in hyperinflators.
Acknowledgements
Funding: This study was funded by the Guangdong Provincial Science and Technology Project (2011B080701062 and 2013B022000072) and Guangdong Natural Science Foundation (S2012010008623).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pepin V, Saey D, Laviolette L, et al. Exercise capacity in chronic obstructive pulmonary disease: mechanisms of limitation. COPD 2007;4:195-204. [PubMed]
- O’Donnell DE, Webb KA. The major limitation to exercise performance in COPD is dynamic hyperinflation. J Appl Physiol (1985) 2008;105:753-5. [PubMed]
- Porszasz J, Emtner M, Goto S, et al. Exercise training decreases ventilatory requirements and exercise-induced hyperinflation at submaximal intensities in patients with COPD. Chest 2005;128:2025-34. [PubMed]
- Casaburi R, Porszasz J, Burns MR, et al. Physiologic benefits of exercise training in rehabilitation of patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997;155:1541-51. [PubMed]
- Chen R, Tian JW, Zhou LQ, et al. The relationship between sleep quality and functional exercise capacity in COPD. Clin Respir J 2014. [Epub ahead of print]. [PubMed]
- Callens E, Graba S, Gillet-Juvin K, et al. Measurement of dynamic hyperinflation after a 6-minute walk test in patients with COPD. Chest 2009;136:1466-72. [PubMed]
- Marin JM, Carrizo SJ, Gascon M, et al. Inspiratory capacity, dynamic hyperinflation, breathlessness, and exercise performance during the 6-minute-walk test in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:1395-9. [PubMed]
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [PubMed]
- Scuarcialupi ME, Berton DC, Cordoni PK, et al. Can bronchodilators improve exercise tolerance in COPD patients without dynamic hyperinflation? J Bras Pneumol 2014;40:111-8. [PubMed]
- Baldi BG, de Albuquerque AL, Pimenta SP, et al. A pilot study assessing the effect of bronchodilator on dynamic hyperinflation in LAM. Respir Med 2013;107:1773-80. [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [PubMed]
- Zheng J, Zhong N. Normative values of pulmonary function testing in Chinese adults. Chin Med J (Engl) 2002;115:50-4. [PubMed]
- O’Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158:1557-65. [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. [PubMed]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377-81. [PubMed]
- Yan S, Kaminski D, Sliwinski P. Reliability of inspiratory capacity for estimating end-expiratory lung volume changes during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997;156:55-9. [PubMed]
- Al-shair K, Dockry R, Mallia-Milanes B, et al. Depression and its relationship with poor exercise capacity, BODE index and muscle wasting in COPD. Respir Med 2009;103:1572-9. [PubMed]
- Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581-6. [PubMed]
- Ringbaek T, Martinez G, Lange P. A comparison of the assessment of quality of life with CAT, CCQ, and SGRQ in COPD patients participating in pulmonary rehabilitation. COPD 2012;9:12-5. [PubMed]
- Hannink J, Lahaije A, Bischoff E, et al. Dynamic hyperinflation after metronome-paced hyperventilation in COPD--a 2 year follow-up. Respir Med 2010;104:1700-5. [PubMed]
- Gagnon P, Guenette JA, Langer D, et al. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2014;9:187-201. [PubMed]
- Leivo-Korpela S, Lehtimäki L, Vuolteenaho K, et al. Adiponectin is associated with dynamic hyperinflation and a favourable response to inhaled glucocorticoids in patients with COPD. Respir Med 2014;108:122-8. [PubMed]
- Zafar MA, Tsuang W, Lach L, et al. Dynamic hyperinflation correlates with exertional oxygen desaturation in patients with chronic obstructive pulmonary disease. Lung 2013;191:177-82. [PubMed]
- Kitahara Y, Hattori N, Yokoyama A, et al. Cigarette smoking decreases dynamic inspiratory capacity during maximal exercise in patients with type 2 diabetes. Hiroshima J Med Sci 2012;61:29-36. [PubMed]
- Stewart JI, Criner GJ. The small airways in chronic obstructive pulmonary disease: pathology and effects on disease progression and survival. Curr Opin Pulm Med 2013;19:109-15. [PubMed]
- O’Donnell DE, Travers J, Webb KA, et al. Reliability of ventilatory parameters during cycle ergometry in multicentre trials in COPD. Eur Respir J 2009;34:866-74. [PubMed]
- Guenette JA, Webb KA, O’Donnell DE. Does dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD? Eur Respir J 2012;40:322-9. [PubMed]
- Kyoh S, Kanazawa H, Tochino Y, et al. Comparison of N-epsilon-(Carboxymethyl) Lysine levels and percentage of eosinophils in induced sputum for assessment of small airway involvements in asthma. Med Sci Monit 2008;14:CR375-8. [PubMed]
- Guillemi S, Wright JL, Hogg JC, et al. Density dependence of pulmonary resistance: correlation with small airway pathology. Eur Respir J 1995;8:789-94. [PubMed]
- Kobayashi D, Tochino Y, Kanazawa H, et al. Comparison of alveolar nitric oxide concentrations using two different methods for assessing small airways obstruction in asthma. Respirology 2011;16:862-8. [PubMed]
- McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011;365:1567-75. [PubMed]
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med 1996;153:976-80. [PubMed]
- van Gestel AJ, Kohler M, Steier J, et al. Cardiac autonomic function and cardiovascular response to exercise in patients with chronic obstructive pulmonary disease. COPD 2012;9:160-5. [PubMed]



