Exponential growth of publications on carbon nanodots by Chinese authors
Introduction
Carbon nanodots (C-dots) is a carbon dominated nanomaterial that holding a discrete and quasi spherical structure, usually containing oxygen/nitrogen organic functional groups (1,2). It was accidently discovered through the purification of single-walled carbon nanotube fragments during electrophoresis in 2004 (1). Over the last decade, C-dots attracted much attention in the areas of bioimaging, biosensing, and drug delivery (3-10). Notably, C-Dots are being developed as fluorescent contrast agents for optical bioimaging (8). Most C-dots are prepared with passivated surface with potential for being further functionalized with other organic and inorganic moieties, and their unique and tunable photoluminescence properties are considered to provide a platform to circumvent the disadvantages of other fluorescent nanomaterials in related applications (11). Although semiconductor quantum dots (QDs) including CdS, CdSe, CdTe have been considered as complementary candidates of fluorochrome and fluorescent proteins, the most serious drawback is the toxic heavy metal elements that usually present in semiconductor QDs, as these heavy metal elements can potentially generate toxicity (12,13).
Worldwide research statistics for C-dots
We looked at the research publication statistics on C-dots from 2004 up till 2014. The Web of ScienceTM search engine was used for investigating publication trend of C-dots in each year, by using the multi-field syntax string “Carbon dots” or “Graphene Quantum Dots (GQDs)” with a timespan from 2004 to 2014. All titles, authors, affiliations, and abstracts were then manually retrieved and analyzed. The trend in publishing topics on molecular imaging was also looked at the top imaging journal Radiology which publishes comprehensive topics of imaging related clinical and experimental studies.
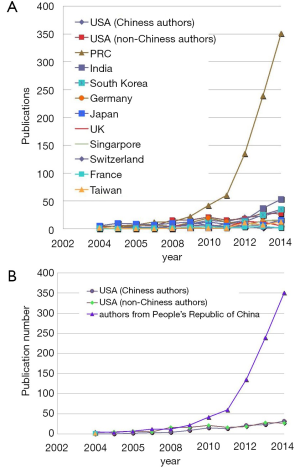
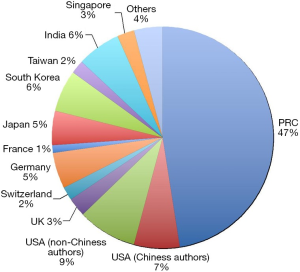
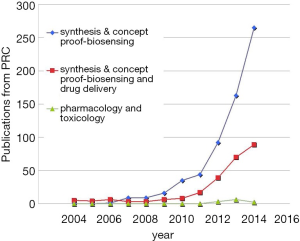
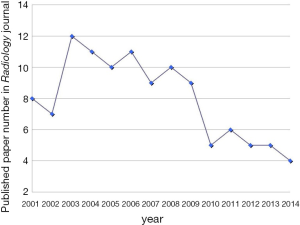
A few clear patterns are evidently demonstrated (Figures 1-3). The number of publications from China mainland authors increased exponentially during this period. Till 2014 China authors contributed 47% of the total publications, by far surpassed USA which is the undisputed leader in science and technology in recent decades. Other major players in pharmaceutical research including Britain (with GlaxoSmithKline and AstraZeneca), Germany (with Bayer), and Switzerland (with Novartis and Hoffman La-Roche), France (with Sanofi) also had a relatively small contribution to the publications. Another issue is that publications by China authors on pharmacology and toxicology lagged far behind publications on chemistry and material science, indicating that research is not solidly moving toward the direction of application (Figure 3). On the other hand, there has been a downward trend in publishing molecular imaging related topics in journal Radiology (Figure 4).
Key obstacles for clinical translation
The difficulties of clinical translation of nanoparticles had been highlighted (14). Many fundamental questions about nanoparticles remain largely unanswered, including how they are in vivo distributed, how they are taken up by the cells, and eliminated in the body. For biomedical imaging, the preferred route of delivery is intravenous injection. However, the average size of capillary pores in normal tissues is only ~5 nm. Thus small molecules equilibrate rapidly between intravascular and extravascular spaces, but nanoparticles do not. Depending on the organic coating surrounding nanoparticles, nonspecific adsorption of plasma proteins, mostly albumin, can also increase effective hydrodynamic diameter. The majority of clearance for small molecules and nanoparticles injected into the bloodstream is through either hepatobiliary or renal route (14). Renal filtration of the blood is mediated by the glomerular basement membrane where the physiologic pore size is ~5 nm in diameter. The physicochemical properties of nanoparticles in serum, such as dispersity, shape, flexibility, and surface charge will also determine whether a nanoparticle can be renally filtered. Nanoparticles larger than the renal filtration threshold will have only three possible fates: metabolism to clearable components, excretion by the liver into bile, or uptake in the reticuloendothelial system therefore resulting in long residence times in the body. Nanoparticles can also sometimes evade detection by the body’s immune system, and under rare circumstances cross the brain blood barrier (15). Even when containing only carbon, certain nanoparticles have been shown to introduce unexpected biological toxicity. In particular, nonbiodegradable nanoparticles that accumulate in certain organs, mostly in the reticuloendothelial, can cause potential harm including immune-mediated toxicity, teratogenicity and carcinogenicity (14-19). For the safety concern, “Choi Criteria” were proposed to guide clinical translation of nanoparticles, namely, degradability minimal non-specific tissue uptake and smaller than 5 nm with renal clearance (14).
Supporting corroborations
Regulatory approvals for nanomaterial-based agents are infrequent (14). Virtually all of the materials are organic, and the only metal-based agent not administered as a microdose is small dextran-coated superparamagnetic iron oxide (SPIO) which is composed of Fe, an element that in moderate quantities is required for red blood cell production. This magnetic resonance imaging contrast agent has been extensively studied (20-22). Still, the history of marketing SPIO nanoparticles illustrates the difficulties of developing nanoparticles for imaging application. SPIO for liver imaging was conceptualized when the speed of both single-slice CT scan and multiple-slice MRI was slow. It was difficult to accurately observe the “wash-in” and “wash-out” of liver lesion blood flow dynamics then. However, spiral CT and later multi-slice CT revolutionized liver imaging (23). MRI scan also became much faster due to the improved gradient technology and fast data acquisition sequence. These techniques increased the sensitivity and specificity of small molecular agent based dynamic CT and MRI imaging. One recent study showed SPIO-enhanced MRI is less efficient than small molecular Gadolinium-enhanced dynamic MRI in the detection and characterization of hepatocellular carcinoma (HCC), due to SPIO-enhanced MRI inability to evaluate pathological vascularity of the nodules (24). Several recent studies demonstrated that MRI using small molecular hepatocyte-specific contrast agent (Gd-EOB-DTPA, Bayer Healthcare) can provide better diagnostic performance for the detection and characterization of HCC. Gd-EOB-DTPA-enhanced liver MRI is currently emerging as a leading method in this regards (25). Together with other reasons, Ferumoxides (Feridex® IV, AMAG Pharma/Endorem®, Guerbet) has been withdrawn from the market, and Ferucarbotran (Resovist®, Bayer Healthycare) being marketed only in limited countries (26). For malignant lymph nodes imaging with the SPIO agent of Ferumoxtran-10 (AMI-227; Combidex®, AMAG Pharma; Sinerem®, Guerbet) particles (27), the pivotal study failed to demonstrate a consistent and statistically significant benefit in sensitivity and failed to confirm non-inferiority with regards specificity (28). Currently only one type of SPIO (ferumoxytol, Ferahaeme®, AMAG Pharma) is marketed for the treatment of treat iron deficiency anaemia in adult patients with chronic kidney diseases, rather than imaging.
For imaging engineering technology outpacing imaging agent, another example is blood pool agent (also known as intravascular contrast agent). Their large size prevents diffusion through the vascular epithelium and leakage into the interstitial space quickly. Blood pool agents remain in the circulation for up to an hour, extending the window available for imaging, allowing better signal-to-noise ratio and improved image resolution for small vasculature. The first agent approved in this class is gadofosveset trisodium (Vasovist®, Lantheus Medical Imaging) (29). Several other agents including Gadocoletic acid (Bracco SpA, Italy), Gadomelitol (Vistarem®, Guerbet) and P792 Gadomer-17 (Bayer Healthcare) had been in development but unlikely will reach the market. The development of very small SPIO particles [VSOP C184, Ferropharm, Germany, (30)] as blood pool contrast agent was also stopped. Actually, small molecular agents based angiography using multi-slice CT or fast MRI reduced the necessity for blood pool contrast agent. Therefore for in vivo nanoparticles imaging agent, the developer has to compete with both the engineering advance of imaging instruments, as well as other smaller molecular agents. This makes the challenges even more daunting.
For optical imaging probes, the limited penetration depth of luminescent or fluorescence contrast is a significant obstacle to in vivo application. To quantify signal strength of luminescent or fluorescence is also known to be problematic (31,32). Optical instruments suitable for small animals will not be suitable for human imaging except for small superficially located lesions which can be easily biopsied or sometime surgically removed. Also note while it has been more than 30 years since the concept of receptor-specific targeted nanoparticles for cancer imaging or treatment was introduced (33), but to date none has been clinically approved. In addition, for nanoparticles each new functionality elevates complexity (e.g., multi-step syntheses, purification and characterization) and cost (e.g., lower yields, more costly materials), and regulatory barriers arise (e.g., owing to multi-component, heterogeneous formulations). As oppose to therapeutic drugs which have a long term access to the market for chronic diseases, the low commercial returns for diagnostic imaging agents, which commonly involve one-time-use in a patient, also hampers clinical development.
Conclusions
Development of imaging agents should have an early involvement of all parties including chemists, pharmacologists, toxicologists, clinicians, pharmas, regulatory authorities, as well as imaging equipment manufacturers (34-36). Cautiously, efforts should be made so that C-Dots research activity should not remain a paper-generating exercise; and the ultimate aim should be the clinical translation to help patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Xu X, Ray R, Gu Y, et al. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc 2004;126:12736-7. [PubMed]
- Sun YP, Zhou B, Lin Y, et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc 2006;128:7756-7. [PubMed]
- Gibbs SL. Near infrared fluorescence for image-guided surgery. Quant Imaging Med Surg 2012;2:177-87. [PubMed]
- Yang ST, Cao L, Luo PG, et al. Carbon dots for optical imaging in vivo. J Am Chem Soc 2009;131:11308-9. [PubMed]
- Lim MC, Seo SS, Kang S, et al. Intraoperative image-guided surgery for ovarian cancer. Quant Imaging Med Surg 2012;2:114-7. [PubMed]
- Dong Y, Wang R, Li G, et al. Polyamine-functionalized carbon quantum dots as fluorescent probes for selective and sensitive detection of copper ions. Anal Chem 2012;84:6220-4. [PubMed]
- Li Y, Hu Y, Zhao Y, et al. An electrochemical avenue to green-luminescent graphene quantum dots as potential electron-acceptors for photovoltaics. Adv Mater 2011;23:776-80. [PubMed]
- Lee JH, Park G, Hong GH, et al. Design considerations for targeted optical contrast agents. Quant Imaging Med Surg 2012;2:266-73. [PubMed]
- Gao X, Yang L, Petros JA, et al. In vivo molecular and cellular imaging with quantum dots. Curr Opin Biotechnol 2005;16:63-72. [PubMed]
- Wang YX. Quantitative Imaging in Medicine and Surgery: progress & perspective. Quant Imaging Med Surg 2013;3:1-4. [PubMed]
- Luo PG, Yang F, Yang ST, et al. Carbon-based quantum dots for fluorescence imaging of cells and tissues. Rsc Adv 2014;4:10791-807.
- Geys J, Nemmar A, Verbeken E, et al. Acute toxicity and prothrombotic effects of quantum dots: impact of surface charge. Environ Health Perspect 2008;116:1607-13. [PubMed]
- Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect 2006;114:165-72. [PubMed]
- Choi HS, Frangioni JV. Nanoparticles for biomedical imaging: fundamentals of clinical translation. Mol Imaging 2010;9:291-310. [PubMed]
- Agarwal A, Lariya N, Saraogi G, et al. Nanoparticles as novel carrier for brain delivery: a review. Curr Pharm Des 2009;15:917-25. [PubMed]
- Warheit DB, Laurence BR, Reed KL, et al. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci 2004;77:117-25. [PubMed]
- Kolosnjaj J, Szwarc H, Moussa F. Toxicity studies of carbon nanotubes. Adv Exp Med Biol 2007;620:181-204. [PubMed]
- Firme CP 3rd, Bandaru PR. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomedicine 2010;6:245-56. [PubMed]
- Stern ST, McNeil SE. Nanotechnology safety concerns revisited. Toxicol Sci 2008;101:4-21. [PubMed]
- Corot C, Robert P, Idée JM, et al. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev 2006;58:1471-504. [PubMed]
- Wang YX. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant Imaging Med Surg 2011;1:35-40. [PubMed]
- Wang YX, Xuan S, Port M, et al. Recent advances in superparamagnetic iron oxide nanoparticles for cellular imaging and targeted therapy research. Curr Pharm Des 2013;19:6575-93. [PubMed]
- Rubin GD. Computed tomography: revolutionizing the practice of medicine for 40 years. Radiology 2014;273:S45-74. [PubMed]
- Kim YK, Kim CS, Kwak HS, et al. Three-dimensional dynamic liver MR imaging using sensitivity encoding for detection of hepatocellular carcinomas: comparison with superparamagnetic iron oxide-enhanced mr imaging. J Magn Reson Imaging 2004;20:826-37. [PubMed]
- Lee JM, Park JW, Choi BI. 2014 KLCSG-NCC Korea Practice Guidelines for the management of hepatocellular carcinoma: HCC diagnostic algorithm. Dig Dis 2014;32:764-77. [PubMed]
- Maurea S, Mainenti PP, Tambasco A, et al. Diagnostic accuracy of MR imaging to identify and characterize focal liver lesions: comparison between gadolinium and superparamagnetic iron oxide contrast media. Quant Imaging Med Surg 2014;4:181-9. [PubMed]
- Heesakkers RA, Jager GJ, Hövels AM, et al. Prostate cancer: detection of lymph node metastases outside the routine surgical area with ferumoxtran-10-enhanced MR imaging. Radiology 2009;251:408-14. [PubMed]
- Withdrawal assessment report for sinerem. European Medicines Agency 2008. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Application_withdrawal_assessment_report/2010/01/WC500067463.pdf
- McGregor R, Vymazal J, Martinez-Lopez M, et al. A multi-center, comparative, phase 3 study to determine the efficacy of gadofosveset-enhanced magnetic resonance angiography for evaluation of renal artery disease. Eur J Radiol 2008;65:316-25. [PubMed]
- Wagner M, Wagner S, Schnorr J, et al. Coronary MR angiography using citrate-coated very small superparamagnetic iron oxide particles as blood-pool contrast agent: initial experience in humans. J Magn Reson Imaging 2011;34:816-23. [PubMed]
- Hoffman RM. Imaging metastatic cell trafficking at the cellular level in vivo with fluorescent proteins. Methods Mol Biol 2014;1070:171-9. [PubMed]
- Close DM, Xu T, Sayler GS, et al. In vivo bioluminescent imaging (BLI): noninvasive visualization and interrogation of biological processes in living animals. Sensors (Basel) 2011;11:180-206. [PubMed]
- Yatvin MB, Kreutz W, Horwitz BA, et al. pH-sensitive liposomes: possible clinical implications. Science 1980;210:1253-5. [PubMed]
- Wang YX. Medical imaging in pharmaceutical clinical trials: what radiologists should know. Clin Radiol 2005;60:1051-7. [PubMed]
- Idée JM, Louguet S, Ballet S, et al. Theranostics and contrast-agents for medical imaging: a pharmaceutical company viewpoint. Quant Imaging Med Surg 2013;3:292-7. [PubMed]
- Corot C, Warlin D. Superparamagnetic iron oxide nanoparticles for MRI: contrast media pharmaceutical company R&D perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2013;5:411-22. [PubMed]


