Urinary excretion of 9α,11β-prostaglandin F2 and leukotriene E4 in patients with exercise-induced bronchoconstriction
Introduction
Exercise-induced bronchoconstriction (EIB) occurs predominantly among patients with asthma. Up to 90% of patients with asthma are thought to experience EIB (1). EIB occurs in some individuals without other features of asthma, with a prevalence of up to 20% (2).
Although the exact mechanism of EIB is not fully understood, hyperosmolarity and cooling of the airway surfaces resulting from hyperpnea is thought to trigger contraction of airway smooth muscle by releasing inflammatory mediators from activated inflammatory cells (3). Several studies have indicated that inflammatory mediators, including histamine, tryptase, leukotrienes (LTs), and prostaglandin (PG) D2 are released into the airway from activated inflammatory airway cells, including eosinophils and mast cells (4-7).
Activation of mast cells has been frequently implicated in the pathogenesis of EIB, as mast cell mediators, such as histamine, tryptase, PGD2, cysteinyl leukotrienes (CysLTs), increase in induced sputum, exhaled breath condensate (EBC), and urine samples of patients with EIB after an exercise challenge test (4,5,8-14). However, the results are inconsistent with other studies, as no increases in histamine, tryptase, PGD2, or CysLTs were detected in bronchial alveolar lavage fluid (BALF) or urine samples (15-18). The preventive effect of disodium cromoglycate or leukotriene receptor antagonists (LTRA) in patients with EIB further supports the importance of these mediators in EIB (4,10,11,19,20).
Eicosanoids, such as PGD2 and CysLTs, act as potent bronchoconstrictors and are important proinflammatory mediators in asthma pathogenesis (21). PGD2 is the major eicosanoid released from activated mast cells and is an established marker of mast cell activation (22). LTE4 is an end metabolite of CysLTs mainly released from mast cells and eosinophils (23). Measuring their metabolites in urine, such as 9α,11β-PGF2 and LTE4, is validated as a marker of allergic inflammation in patients with asthma (22,23), and collecting urine is a simple, convenient and noninvasive sampling technique (23). A few studies have determined urinary excretion of both 9α,11β-PGF2 and LTE4 together in patients with EIB. One UK study group demonstrated increased urinary 9α,11β-PGF2 and LTE4 in elite athletes with EIB (12), whereas a Swedish group did not find an increase in urinary LTE4 levels after exercise in adult patients with asthma, but found an increase in urinary 9α,11β-PGF2 (9).
The aim of this study was to investigate the role of mast cell activation in patients with EIB by measuring urinary excretion of 9α,11β-PGF2 and LTE4 before and after an exercise challenge test in adult patients with asthma and EIB and in normal controls.
Materials and methods
Study subjects
Eight patients with a history of EIB and five normal controls were enrolled retrospectively. Each subject performed an exercise challenge test. Eight of the study subjects were positive responders, and five were non-responders. All patients with EIB had mild asthma. A diagnosis of asthma was established based on either a positive response to methacholine challenge test (PC20 <25 mg/mL) or a positive response to the mannitol bronchoprovocation test (PD15 <635 mg). One patient with EIB had physician-diagnosed asthma for 3 years. He refused to perform a test for asthma diagnosis. Subjects were excluded if baseline forced expiratory volume in 1 s (FEV1) was ≤70% of the predicted value, if the patient was treated for asthma exacerbation within the previous month, or if the subject had parenchymal lung disease on chest radiography.
Seven subjects had atopy, as diagnosed by at least one positive reaction to allergy skin prick tests or multiple simultaneous allergen tests (AdvanSure AlloScreen®, LG, Seoul, Korea) which included common inhalant allergens, such as Dermatophagoides pteronyssinus, Dermatophagoides farinae, tree pollen, grass pollen, ragweed, mugwort, Alternaria, and animal dander. This study was approved by the local Institutional Review Board, and written informed consent was obtained from all participants.
Spirometry, methacholine and mannitol bronchial provocation
Spirometry and methacholine challenges were conducted in accordance with the guidelines of the American Thoracic Society (24). Spirometry was performed with a VmaxTM (Sensormedics, Milan, Italy). Mannitol bronchial provocation was conducted according to the manufacturer’s instructions (Aridol®, Pharmaxis Ltd. Frenchs Forest, NSW, Australia). Inhaled corticosteroids, leukotriene modifiers, or any bronchodilators (short- or long-acting inhaled β2 agonists, methylxanthines, etc.) were stopped at least 1 week before the spirometry and bronchial provocation tests.
Exercise challenge
Exercise challenges were performed at least 1 week apart from the methacholine or mannitol bronchial provocation tests. Inhaled corticosteroids, leukotriene modifiers, or any bronchodilators (short- or long-acting inhaled β2 agonists, methylxanthines, etc.) were stopped at least 1 week before the exercise challenge. The protocol mostly followed the standardized guidelines of the American Thoracic Society (24). Exercise challenges were performed by either free-running or treadmill exercise for 6 min at ≥85% of maximum heart rate. Each subject wore a nose clip during the exercise challenge. Spirometry was conducted before and 3, 6, 9, 12, 15, and 30 min after exercise. A positive response was defined ≥15% decrease in FEV1.
Measurement of urinary 9α,11β-PGF2 and LTE4 concentrations
Urine was collected before and 1 h after the end of the exercise challenge test. The urine samples were stored at −20 °C until analysis. The 9α,11β-PGF2 and LTE4 were measured by enzyme immunoassay (EIA) using an EIA kit according to the manufacturer’s instructions (Cayman Chemical Company, Ann Arbor, MI, USA). Urinary excretion of 9α,11β-PGF2 and LTE4 is expressed as ng/mmol creatinine. Creatinine was measured in all urine samples using a commercially available colorimetric assay (Sigma-Aldrich, St. Louis, MO, USA).
Statistical analysis
All data are expressed as means ± standard deviation (SD). Mean values of the mediators between the two groups were compared using the Mann-Whitney U-test. Differences in the mediators before and after exercise were compared using the Wilcoxon signed-rank test. The correlation between percent decrease in FEV1 and percent change in the mediators during the exercise challenge test was analyzed using Spearman’s correlation analysis. A P value <0.05 was considered to indicate significance. All statistical analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Results
The clinical characteristics of the study subjects are summarized in Table 1. Mean age was 19.7±1.5 years (range, 17-22 years), and all subjects were male. Two of the subjects were smokers (<2 pack-year). No significant differences were observed in age, sex, or body mass index between the patients with EIB and controls. Five of the eight patients with EIB had atopy. Baseline FEV1 (% predicted) was significantly lower in patients with EIB than that in controls (91.1±17.7 vs. 96.6±17.0, P=0.02), and baseline FEV1/forced vital capacity (%) was also lower in patients with EIB than that in the control group (70.5±10.0 vs. 83.2±6.9, P<0.05). The maximum percent decrease in FEV1 after exercise was 23.0%±8.0% in patients with EIB, whereas it was 5.6%±2.3% in normal controls (P<0.05).
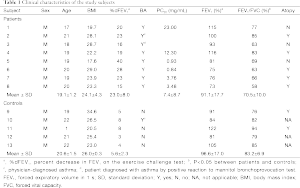
Full table
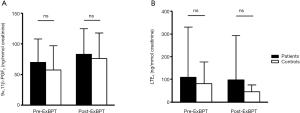
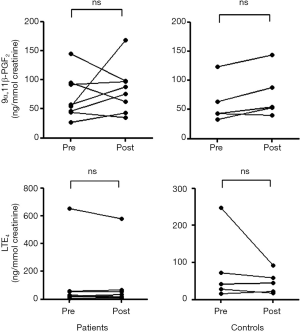
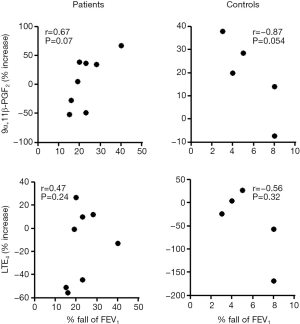
No significant difference was observed in the baseline 9α,11β-PGF2 level between patients with EIB and controls (70.2±38.0 vs. 61.4±36.3 ng/mmol creatinine), and no significant difference was observed in the 9α,11β-PGF2 level after the exercise challenge between patients with EIB and controls (83.5±41.4 vs. 76.1±41.7 ng/mmol creatinine) (Figure 1). No difference was observed in the urinary LTE4 level before (109.7±220.5 vs. 81.7±95.5 ng/mmol creatinine) or after the exercise challenge (99.5±195.2 vs. 47.3±30.3 ng/mmol creatinine) between patients with EIB and controls. Urinary excretion of 9α,11β-PGF2 in patients with EIB did not increase significantly after the exercise challenge (Figure 2). Urinary excretion of LTE4 also did not significantly change after the exercise challenge. The maximum percent decrease in FEV1 during the exercise challenge test in patients with EIB tended to be positively correlated with the percent change in 9α,11β-PGF2 level (r=0.67, P=0.07) (Figure 3). The percent change in LTE4 in patients with EIB was not correlated with the maximum percent decrease in FEV1 on the exercise challenge test (r=0.47, P>0.05).
Discussion
In this study, we did not detect an increase in urinary 9α,11β-PGF2 or LTE4 levels after an exercise challenge test in patients with EIB, and urinary levels of 9α,11β-PGF2 and LTE4 after the exercise challenge in patients with EIB were not significantly higher than those in a control group. In addition, the percent decrease in FEV1 during the exercise challenge test was not correlated with the percent changes in 9α,11β-PGF2 and LTE4 levels.
Activation of mast cells has long been implicated in the pathogenesis of EIB, as plasma histamine increases after exercise in patients with EIB, and the mast cell stabilizer disodium cromoglycate attenuates EIB (19). Histamine and tryptase release in induced sputum from EIB patients was also noted following an exercise challenge (4). Crimi et al. showed that the number of degranulated mast cells increases in bronchial biopsies from patients with EIB after an exercise challenge (7). However, other studies have not demonstrated an increase in histamine in plasma, BALF, bronchial lavage, or urine after exercise in patients with EIB, suggesting that mast cells may not contribute to the mechanism of EIB (9,15,16,25). Eosinophilic airway inflammation is also detected in patients with EIB, as eosinophils and eosinophil cationic proteins increase in induced sputum or BALF after exercise (5-7). However, Gauvreau et al. did not demonstrate eosinophilic airway inflammation after exercise in patients with EIB (26).
Eicosanoids, such as PGD2 and CysLTs, are released from activated mast cells or eosinophils and are frequently isolated from induced sputum, BALF, and urine to investigate their roles in EIB. However, previous results have been inconsistent. Interestingly, significant increases in PGD2 and/or CysLTs have been consistently observed in induced sputum and EBC (4-6,13), but not in BALF (15). Because EIB is initiated by drying and cooling of the airway, which causes bronchoconstriction in the segmental airways (27), BALF collected from the distal airway may not be appropriate to identify relevant airway inflammation.
Urinary LTE4 and 9α,11β-PGF2 levels are widely used as a marker of allergic inflammation (22,23). However, urinary excretion results of LTE4 and 9α,11β-PGF2 in patients with EIB vary among studies. Several studies that have measured urinary LTE4 failed to show a significant increase after exercise in patients with EIB, including the current study (9,17,18), whereas other studies demonstrated increase of urinary LTE4 (10-12,28). In contrast, urinary 9α,11β-PGF2 levels in previous studies increased significantly and consistently in patients with EIB after exercise (8,9,12). However, we could not demonstrate a significant increase in urinary 9α,11β-PGF2 level in patients with EIB, although the percent change in 9α,11β-PGF2 level tended to be positively correlated with the maximum percent decrease in FEV1 during the exercise challenge test in patients with EIB (r=0.67, P=0.07) (Figure 3).
Several explanations are possible for these discrepancies among studies. First, individual variations in release of eicosanoids during exercise, such as race, age, sex, or body mass index may occur among patients with EIB. Second, the timing of urine collection may have influenced eicosanoid levels. Too early or too late a urine collection may have caused negative results. According to the kinetics of LTE4 excretion, the majority of urinary LTE4 is excreted within 2-4 h after allergen challenge or inhalation of LTD4 (29,30). In this study, we collected urine 1 h after the end of the exercise challenge test, which may have been too early to detect urinary excretion of LTE4 and/or 9α,11β-PGF2 after exercise. However, two studies with positive results determined LTE4 and/or 9α,11β-PGF2 within 1-2 h after an exercise challenge (11). One study serially collected urine at 0-3, 3-6, 6-12, and 12-24 h after an exercise challenge, but did not show an increase in urinary LTE4 at any time point (18). Furthermore, Park et al. did not demonstrate increased urinary excretion of LTE4 6 h after exercise in Korean patients with EIB (28). Taken together, these results suggest that our timing of urine collection after the exercise challenge may not influence the negative results. Third, total release of LTS from the airway after an exercise challenge may be too low to detect in systemic circulation and/or urine. It is also possible that locally produced mediators were trapped in areas of poor perfusion in response to airway cooling, resulting in poor absorption into systemic circulation (18). The release of urinary LTE4 after an exercise challenge is about twofold higher than that at baseline (9,17,18), whereas a 4- or 12-fold increase in urinary LTE4 has been reported after allergen challenge (18,30). In addition, when we measured urinary 9α,11β-PGF2 in a patient with exercise-induced anaphylaxis as a positive control of mast cell activation, there was about 12-fold increase of urinary 9α,11β-PGF2 after exercise challenge (80.1 vs. 998.9, data not shown). Thus, the role of mast cell activation in patients with EIB may be feeble compared to that during allergen provocation or systemic allergic reactions such as anaphylaxis. This explanation also agrees with the consistent increase in inflammatory mediators in induced sputum and EBC after an exercise challenge (4-6,13,14).
Mechanisms other than inflammatory mediator release from activated mast cells or eosinophils may be involved in EIB pathogenesis. Airway epithelial injury by thermal or osmotic stress decreases PGE2 production, which has anti-inflammatory and bronchodilatory effects in patients with asthma (31). An increase in the CysLTs/PGE2 ratio has been observed in induced sputum from patients with EIB after an exercise challenge (6). Sensory neuronal activation by electrical and chemical stimuli has been reported to be another mechanism of EIB (32).
Our study had several limitations. We had a relatively small number of subjects with heterogeneous clinical characteristics of atopy and smoking history; the exercise challenge protocols were inconsistent among the subjects, such as free-running or treadmill exercise; and we did not evaluate other mast-cell-specific inflammatory mediators such as histamine and tryptase.
Conclusions
Our findings do not support a role of inflammatory mediators such as PGD2 and CysLTs, which are known as a marker of mast cell and eosinophil activation, in Korean patients with EIB. As activation of inflammatory cells in EIB was evidenced by an increase of inflammatory mediators in induced sputum or EBC in other studies (4-6,13,14), measuring urinary excretion of 9α,11β-PGF2 and LTE4 may not be a good marker for detection of airway inflammation in patients with EIB.
Acknowledgements
Funding: This study was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF), as funded by the Ministry of Education, Science, and Technology (2012R1A1A1012349).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Storms WW. Review of exercise-induced asthma. Med Sci Sports Exerc 2003;35:1464-70. [PubMed]
- Weiler JM, Bonini S, Coifman R, et al. American Academy of Allergy, Asthma & Immunology Work Group report: exercise-induced asthma. J Allergy Clin Immunol 2007;119:1349-58. [PubMed]
- Anderson SD, Daviskas E. The mechanism of exercise-induced asthma is…. J Allergy Clin Immunol 2000;106:453-9. [PubMed]
- Hallstrand TS, Moody MW, Wurfel MM, et al. Inflammatory basis of exercise-induced bronchoconstriction. Am J Respir Crit Care Med 2005;172:679-86. [PubMed]
- Mickleborough TD, Lindley MR, Ray S. Dietary salt, airway inflammation, and diffusion capacity in exercise-induced asthma. Med Sci Sports Exerc 2005;37:904-14. [PubMed]
- Hallstrand TS, Moody MW, Aitken ML, et al. Airway immunopathology of asthma with exercise-induced bronchoconstriction. J Allergy Clin Immunol 2005;116:586-93. [PubMed]
- Crimi E, Balbo A, Milanese M, et al. Airway inflammation and occurrence of delayed bronchoconstriction in exercise-induced asthma. Am Rev Respir Dis 1992;146:507-12. [PubMed]
- Nagakura T, Obata T, Shichijo K, et al. GC/MS analysis of urinary excretion of 9alpha,11beta-PGF2 in acute and exercise-induced asthma in children. Clin Exp Allergy 1998;28:181-6. [PubMed]
- O’Sullivan S, Roquet A, Dahlén B, et al. Evidence for mast cell activation during exercise-induced bronchoconstriction. Eur Respir J 1998;12:345-50. [PubMed]
- Kikawa Y, Miyanomae T, Inoue Y, et al. Urinary leukotriene E4 after exercise challenge in children with asthma. J Allergy Clin Immunol 1992;89:1111-9. [PubMed]
- Reiss TF, Hill JB, Harman E, et al. Increased urinary excretion of LTE4 after exercise and attenuation of exercise-induced bronchospasm by montelukast, a cysteinyl leukotriene receptor antagonist. Thorax 1997;52:1030-5. [PubMed]
- Mickleborough TD, Murray RL, Ionescu AA, et al. Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am J Respir Crit Care Med 2003;168:1181-9. [PubMed]
- Carraro S, Corradi M, Zanconato S, et al. Exhaled breath condensate cysteinyl leukotrienes are increased in children with exercise-induced bronchoconstriction. J Allergy Clin Immunol 2005;115:764-70. [PubMed]
- Bikov A, Gajdócsi R, Huszár É, et al. Exercise increases exhaled breath condensate cysteinyl leukotriene concentration in asthmatic patients. J Asthma 2010;47:1057-62. [PubMed]
- Broide DH, Eisman S, Ramsdell JW, et al. Airway levels of mast cell-derived mediators in exercise-induced asthma. Am Rev Respir Dis 1990;141:563-8. [PubMed]
- Jarjour NN, Calhoun WJ. Exercise-induced asthma is not associated with mast cell activation or airway inflammation. J Allergy Clin Immunol 1992;89:60-8. [PubMed]
- Taylor IK, Wellings R, Taylor GW, et al. Urinary leukotriene E4 excretion in exercise-induced asthma. J Appl Physiol (1985) 1992;73:743-8. [PubMed]
- Smith CM, Christie PE, Hawksworth RJ, et al. Urinary leukotriene E4 levels after allergen and exercise challenge in bronchial asthma. Am Rev Respir Dis 1991;144:1411-3. [PubMed]
- Lee TH, Brown MJ, Nagy L, et al. Exercise-induced release of histamine and neutrophil chemotactic factor in atopic asthmatics. J Allergy Clin Immunol 1982;70:73-81. [PubMed]
- Manning PJ, Watson RM, Margolskee DJ, et al. Inhibition of exercise-induced bronchoconstriction by MK-571, a potent leukotriene D4-receptor antagonist. N Engl J Med 1990;323:1736-9. [PubMed]
- Boyce JA. Eicosanoids in asthma, allergic inflammation, and host defense. Curr Mol Med 2008;8:335-49. [PubMed]
- Dahlén SE, Kumlin M. Monitoring mast cell activation by prostaglandin D2 in vivo. Thorax 2004;59:453-5. [PubMed]
- Kumlin M. Measurement of leukotrienes in humans. Am J Respir Crit Care Med 2000;161:S102-6. [PubMed]
- Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 2000;161:309-29. [PubMed]
- Hartley JP, Charles TJ, Monie RD, et al. Arterial plasma histamine after exercise in normal individuals an in patients with exercise-induced asthma. Clin Sci (Lond) 1981;61:151-7. [PubMed]
- Gauvreau GM, Ronnen GM, Watson RM, et al. Exercise-induced bronchoconstriction does not cause eosinophilic airway inflammation or airway hyperresponsiveness in subjects with asthma. Am J Respir Crit Care Med 2000;162:1302-7. [PubMed]
- Kotaru C, Coreno A, Skowronski M, et al. Morphometric changes after thermal and methacholine bronchoprovocations. J Appl Physiol (1985) 2005;98:1028-36. [PubMed]
- Park JK, Bahn JW, Lee BJ, et al. Urinary N-methylhistamine and sulfidopeptide leukotriene in exercise-induced asthma. J Asthma 1998;18:40-51.
- Verhagen J, Bel EH, Kijne GM, et al. The excretion of leukotriene E4 into urine following inhalation of leukotriene D4 by human individuals. Biochem Biophys Res Commun 1987;148:864-8. [PubMed]
- Manning PJ, Rokach J, Malo JL, et al. Urinary leukotriene E4 levels during early and late asthmatic responses. J Allergy Clin Immunol 1990;86:211-20. [PubMed]
- Holgate ST, Peters-Golden M, Panettieri RA, et al. Roles of cysteinyl leukotrienes in airway inflammation, smooth muscle function, and remodeling. J Allergy Clin Immunol 2003;111:S18-34; discussion S34-6.
- Kippelen P, Anderson SD. Pathogenesis of exercise-induced bronchoconstriction. Immunol Allergy Clin North Am 2013;33:299-312. [PubMed]


