Fibrinous tumor of the pleura: an orphan disease lost in translation
Abstract
Fibrous dysplasia is an uncommon, benign disorder also known as fibrous mesothelioma. The cause of fibrous dysplasia is unknown. They represent 5% of all pleura neoplasms and in 80% of all cases arise from the visceral pleura. The epidemiology of the disease is reported equal between males and females around the age of 50. Fibrous dysplasia is usually asymptomatic, although several disease symptoms have been reported as hypoglycemia, pain and swelling may accompany the lesion, in advanced disease. Chemotherapy has not presented disease control; nevertheless, radiotherapy is efficient and indicated in residual disease. The disease progress is usually benign; however several disease manifestations have been reported. There are several molecular pathways, which are possible activated during the disease progress and therefore the disease expression changes throughout its course.
Key words: Fibrinous tumor; molecular pathways; fibrous mesothelioma
Introduction
The solitary fibrinous tumor of the pleura (SFTP) is a very interesting albeit relatively uncommon soft-tissue tumor. It is a localized, usually benign, primary neoplasm of the pleura, the origin of which was a controversial issue for many years. In the past, a variety of terms, such as benign localized pleural mesothelioma, pleural fibroma, submesothelial fibroma, subserosal fibroma, localized fibrous mesothelioma or localized fibrous tumor was used to describe this pathological entity (1-4). Due to advances in immunohistochemistry and electron microscopy, a number of details have been clarified so that nowadays this primary pleural neoplasm has acquired a specific name and status according to its correct histogenesis. However, additional markers that are not always investigated, such as the c-peptide and cortisol, might add to the diagnostic work up of the clinical expression and disease manifestation. Several markers such as; c-peptide, cortisol, insulin and blood glucose were carefully collected from three different cases and were associated with individual patient disease.
Case series
Case 1
A 50-year-old Caucasian female, who complained for right shoulder and cervical pain visited an orthopedic doctor, who recommended her an MRI examination of the cervix and the right shoulder. The MRI revealed a mass in the right upper lobe, a mass in the right hemithorax with unspecified borders to the spine and to the lung Figure 1. She was advised to visit the pneumological medical practice. She was a smoker (15 packyears). The physical examination revealed a reduction of breath sounds in the right hemithorax, her saturation was 97% and her blood gas count was PO2=90 mmHg and PCO2=35.5 mmHg. Afterwards, she was submitted to bronchoscopy. The lung lavage was examined for cancer cells and the examination was negative. The cytologic examination of the lavage was also negative for bacillus Tuberculosis and showed only inflammatory cells. The patient was submitted to a CT guided lung biopsy and a tissue sample was taken from the tumoral mass located in the right apical lobe with 1.2 cm length Figure 1. The examination of the sample showed increased cellularity with a few polymorphisms. The fluoroimmunoassay demonstrated that the sample was negative for CD34, Bcl-2, S100, SMA and vimentin, results more compatible with solitary SFTP.
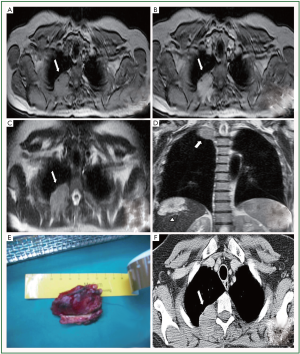
The patient was taken to the operation room (OR) and the tumor was resected on healthy boundaries at the upper tumor boundaries and on boundaries that included the resection field at the part of the tumor that was located on the spine at the part of the third thoracic rib with 4 cm length. The part of the tumor that was resected was measured and its dimensions were 5 cm × 1.2 cm and the histopathological examination showed a fibrinous pleura tumor Figure 1. The immunohistochemistry was positive for Bcl-2, SMA and vimentin and it was negative for CD34, CD33, S100 and calretinin. The patient recovered well after two days in the Intensive Care Unit. Based on the increased mitotic activity observed in the surgically resected tumor (>8/10 hpf). Additional, postoperative radiotherapy fractionated in six fractions of 10 Gy was initiated. Her post operative check included CTs of the brain, upper and lower abdomen, retroperitoneal area, bone scan, mastography and a breast ultrasound. Blood test revealed elevated SFT markers pre- and post-surgery Table 1.

Full Table
Case 2
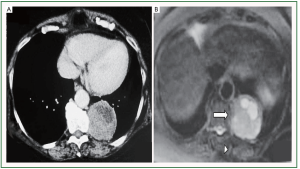
The second patient is a 60 year old Caucasian woman who visited a cardiologist for her annual check-up. The patient had a medical history of hypertension, dyslipidemia for the last 10 years, hyperuricemia and gastro-oesophageal reflux the last 5 years. She is under medication irbesartan and hydrochlorothiazide 325 mg, allopurinol 300 mg, pravastatin 40 mg once a day and omega 3 acid-ethyl-esters 1 g twice a day. The cardiological evaluation was normal, with no pathological findings at the echocardiography, Doppler heart examination and the stress test. The patient complained a mild thoracic pain and therefore she was advised to have a contrast dye thorax CT scan Figure 2. The examination revealed a non-pulmonary spheric mass located in the posterior midiastinum with dimensions 55 mm × 55 mm. The patient was referred to a pulmonary department. The physical examination was normal and the doctors recommended her to have a thorax MRI Figure 2. The MRI revealed a well-shaped, spindle, soft tissue mass in the posterior basal lobe of the left lung with wide base to the pleura, near the spine with dimensions 7.2 cm × 6.2 cm × 5.6 cm. Those findings are more compatible with SFTP. The patient was submitted to bronchoscopy. The lavage cytological examination was negative for cancer cells and positive for inflammatory cells. A CT guided lung biopsy followed the bronchoscopy. The findings from the examination were indicative for SFTP. The patient was taken to the operation room and a tumor measured 7.2 cm × 6.2 cm × 5.6 cm was resected on healthy boundaries. The histological examination of the resected tumor confirmed the diagnosis of SFTP. The patient’s 6 month follow up is disease free. Blood test revealed mildly elevated SFT markers which decreased after surgery Table 1.
Case 3
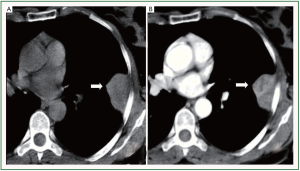
The third patient is a 56 year old Caucasian female, who presented in the Emergency room, because of acute thoracic pain. The patient had no medical record. By the time of her acceptance she had a negative troponine test, a normal ECG and a heart echo test that was also normal. She had a thorax X-ray, which revealed a radiopaque well defined mass in the left median lung field with dimension 5 cm × 3.5 cm. A thorax CT was performed and revealed a 4 cm × 4.5 cm × 2.8 cm mass located in the left hemithorax that was lying on the lateral thoracic wall, the pleura and the major fissure corpus callosum Figure 3. The patient was referred to a pulmonary department. The patient was submitted to bronchoscopy, and alveolar washing, the lavage cytological examination was negative for cancer cells and positive for inflammatory cells. A CT guided lung biopsy was performed. The examination of the biopsy sample showed increased cellularity with a few polymorphisms. The fluoroimmunoassay demonstrated that the sample was negative for CD34, Bcl-2, S100, SMA and Vimentin, results more compatible with solitary SFTP. The patient was submitted to a surgical tumorectomy. The histopathology examination of the resected tumor, that followed, was negative for malignancy and compatible with SFTP. The patient is healthy after the six month follow up. Blood test of SFT markers revealed normal values Table 1.
Discussion
SFTP was probably first mentioned by Wagner in 1870, but the first description of its pathology appeared in 1931 by Klemperer and Rabin (1-5). It is a rare neoplasm that represents 5% of all pleural neoplasms (4). Nowadays, it is known that SFTP arises from mesenchymal cells in the areolar tissue subjacent to the mesothelial-lined pleura and is most often detected primarily in visceral pleura (in 80% of the cases) (4). Nevertheless, because of its mesenchymal cell origin, it may also derive from the parietal pleura and other extrathoracic locations such as the meninges, nose, oral cavity, pharynx, epiglottis, salivary gland, thyroid, breast, kidney, bladder, spinal cord (1), pericardium and peritoneum (4).
It mainly occurs in the 6th and 7th decade of life and has the same frequency in both sexes. There is no apparent genetic predisposition and, unlike the malignant diffuse mesothelioma, SFTP has no relationship to asbestos exposure, tobacco, or any other environmental agent. The size of the tumor varies between 1-36 cm (mean 6 cm) in diameter. It usually appears as a well-delineated, lobulated mass that is heterogenous in attenuation (low attenuation corresponds to areas of hemorrhage or central necrosis). The tumor most commonly arises as a pedunculated mass so it may be mobile within the pleural cavity, or as a sessile lesion which abuts the pleural surface by a broad base and is located near the lung periphery (1). Numerous thin-walled vessels may be present in larger tumors (4).
According to the literature, 50% of the reported cases show that the disease appears as an asymptomatic mass and is usually discovered incidentally on a chest X-ray. Symptoms tend to appear more often in larger lesions and are categorized as thoracic, such as chest pain, cough, dyspnoea and hemoptysis, or general symptoms, such as weakness, nocturnal sweating, chills, weight loss (4). Paraneoplastic syndromes may also occur in a patient with SFTP. Hypertrophic osteoarthropathy (Pierre-Marie-Bamberg syndrome) is the most common paraneoplastic syndrome. It is characterized by finger clubbing, which is associated with hypertrophic skin changes and periosteal bone changes. It has been reported in 20% of the cases and is thought to be caused by increased production of hyaluronic acid by the tumor cells. The pathophysiology of finger-clubbing remains unascertained (2,4). Hypoglycemia (Doege-Potter syndrome), which is observed in approximately 5% of cases, results from the secretion of an unprocessed or incomplete, high molecular weight (HMW) form of insulin-like growth factor type II (IGF-II) which lowers the blood glucose levels (2). Pleural effusion related to this tumor accounts for fewer than 10% of all cases.
The chest computed tomography (CT) scan is the key examination which shows more clearly the size and location of the tumor. Nevertheless, imaging tests (chest X-Ray and CT) are not specific and adequate for the pre-operative diagnosis of SFTP. Moreover, they cannot differentiate between benign and malignant lesions. CT-guided fine needle aspiration provides the diagnosis in many cases. Magdeleinat et al. (6) reported an accuracy rate of 45%, which was similar in that reported by Sung et al. (7). A confident pre-operative diagnosis can be made by a histologic and immunohistochemical analysis of material obtained by a transthoracic Tru-Cut needle biopsy (4). Sometimes a magnetic resonance imaging (MRI) is useful in evaluating the potential invasion of adjacent tissues (chest wall, diaphragm, and mediastinum). The Positron-emission tomography (PET) is a noninvasive technique used to differentiate benign from malignant masses, however, its usefulness remains a controversial issue in the literature (1-4).
This neoplasm (SFT) is usually found in soft tissues and it has been observed until now in several organs including breast, peritoneum, salivary gland, pericardium, liver, mediastinum, and upper respiratory tract including nasal cavity (8-12).
The morphology is usually described as of plump or slender spindle or rounded cells in a collagenous background. Mitotic activity varies from 0-50 per 10 hpf, and some cases have necrosis or hemorrhage. Histology views SFTP as a fibrous and myofibroblastic spindle cell neoplasm. The “patern-less” patern is the most common, followed by the hemangiopericytoma pattern (2,4). Immunohistochemically, diffuse of CD-34 and bcl-2 expression is a feature of this tumor type observed (13-15). In case of CD34-negative tumor in an extra-pleural location the diagnosis should not be solitary fibrous tumor unless the other evidence is compelling (8). CD-99 is also positive and sporadically there is focal positivity for actin, S100 protein and (exceptionally) cytokeratin. Fibroblastic cells are observed with very occasional myofibroblasts in electron microscopy. The SFT does not show evidence of mesothelial differentiation, despite of initial reports. Several genetic findings have been reported including loss of 13q, 4q and 21q; trisomy 21; and gains at chromosome 8 and at 15q; [4;15] [q13;q26] (16), t[6;17] [p11.2;q23] (17), and t [9;22] [q31;p13] (18), and also, once again, rearrangements involving 12q13-15 (17). Myxoid SFT change exceeding 50% of the tumor represents <5% of SFT (19). Vascular myxoid stroma, with focal cellular aggregates, collagenization and staghorn vessels are common in the tissue observation. Nuclear atypia is absent, except in the case of malignancy transformation. In all cases CD-34 and CD-99 are positive; in the case of negative CD-34 malignancy manifestation might be observed in the clinical course of the disease. Recurrence is more likely with larger and histologically aggressive tumors (increased mitosis >4/10 hpf and loss of CD-34 expression), development of fibrous sarcoma is also possible in the case of malignant transformation (8). The vascular endothelial growth factor (VEGF) and platelet derived growth factor pathway (PDGF) is observed overexpressed when hyperthrophic osteoarthropathy is observed.
The SFT cells have a markedly greater expression of IGF-II mRNA and a lesser expression of pro-hormone convertase 4 [PC 4] mRNA in the tumor tissue compared to normal placental tissue. Pro-hormone convertase 4 is an endoprotease involved in processing precursor HMW IGF-II that cleaves pro-IGF-II to generate the mature IGF-II (20). Defective PC4 gene expression in these tumors may underlie the impaired processing of IGF-II (21,22). The unprocessed HMW IGF-II has higher bioavailability, compared to process IGF-I, IGF-II and insulin, nevertheless, the bind with the serum is decreased. Therefore increased levels of free HMW IGF-II are observed (23,24). However, some of these paraneoplastic hypoglycemias in carcinomas are attributed to IGF-I rather than IGF-II. It appears from our measurements of C-peptide, cortisol, insulin and blood glucose that the values depended of the tumors biological expression. In the first case where malignant transformation occurred based on the histological examination the markers were initially elevated and remained elevated after tumor resection. In the second case the markers were initially slightly elevated and decreased after surgery, and in the third case the values did not change significantly Table 1. It has not been observed if post-surgery radiation/chemotherapy play a role in the biological expression of the previously stated tumor markers. Tumor neo-adjuvant pretreatment with chemotherapy and radiotherapy has demonstrated disease control, and in addition radiotherapy is an added as port-surgery adjuvant treatment in the case of residual disease. Nevertheless, despite the indicated treatment recurrence of the disease is often and the possibility of malignant transformation exists.
Despite its benign behavior, SFTP may also show malignancy (local recurrence and metastases). It is reported that the malignant tumors are usually greater than 10 cm in diameter (3). England et al. (25) reviewed criteria for malignancy and included abundant cellularity, more than four mitoses per ten high-power fields, cytonuclear atypia, large necrotic or hemorrhagic areas, an associated pleural effusion, atypical location and invasion of adjacent structures. Using these criteria, 12-33% of SFTP were considered to be malignant. However, neither the size nor these unfavorable histological features must be considered as mandatory diagnostic criteria for malignancy (1). A supernumerary chromosome 8 suggests a more malignant behavior of SFTP, but cytogenetic data are sparse and there is not enough evidence (4).
The mainstay of treatment of SFTP is a surgical resection. Open thoracotomy is the preferred technique in order to remove large masses. Small peduncunlated lesions (diameter <5 cm) located on the visceral pleura can be safely removed by VATS. In any case, the surgeon must obtain histologic negative margins so as to avoid recurrence. Adjuvant treatment (radiotherapy and chemotherapy) is of limited value. Radiotherapy is used in residual disease, as well as for recurrences (2). Due to the rarity of these tumors, there is no systematic assessment of the treatment role.
Local recurrence rate of SFTP is mainly depended on the completeness of the surgical treatment. Moreover, De Perrot et al. (26) provided a classification according to tumor characteristics and prognosis: (I) Benign pedunculated tumors have a 2% recurrence rate; (II) Benign sessile tumors have an 8% recurrence rate; (III) Malignant pedunculated tumors have a 14% recurrence rate; (IV) Malignant sessile tumors have a 63% recurrence rate and 30% mortality. CT scans should be used to monitor possible recurrence every 6 months for the first 2 years and then yearly. Most recurrences occur within 24 months of the initial resection. Nevertheless, all SFTP patients need long-term follow-up (15-20 years) due to the possibility of late recurrences (1,4).
In our three cases several markers were investigated in order observe a possible connection between disease status upon diagnosis and upon follow up. Based on the data we collected the markers such as; c-peptide, cortisol, insulin and blood glucose present useful information regarding disease status and prognosis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Robinson LA. Solitary fibrous tumor of the pleura. Cancer Control 2006;13:264-9.
- Kalebi AY, Hale MJ, Wong ML, et al. Surgically cured hypoglycemia secondary to pleural solitary fibrous tumour: case report and update review on the Doege-Potter syndrome. J Cardiothorac Surg 2009;4:45.
- Guo W, Xiao HL, Jiang YG, et al. Retrospective analysis for thirty-nine patients with solitary fibrous tumor of pleura and review of the literature. World J Surg Oncol 2011;9:134.
- Thakkar RG, Shah S, Dumbre A, et al. Giant solitary fibrous tumour of pleura -an uncommon intrathoracic entity- a case report and review of the literature. Ann Thorac Cardiovasc Surg 2011;17:400-3.
- Lu C, Ji Y, Shan F, et al. Solitary fibrous tumor of the pleura: an analysis of 13 cases. World J Surg 2008;32:1663-8.
- Magdeleinat P, Alifano M, Petino A, et al. Solitary fibrous tumors of the pleura: clinical characteristics,surgical treatment and outcome. Eur J Cardiothorac Surg 2002;21:1087-93.
- Sung SH, Chang JW, Kim J, et al. Solitary fibrous tumors of the pleura: surgical outcome and clinical course. Ann Thorac Surg 2005;79:303-7.
- Chan JK. Solitary fibrous tumour--everywhere, and a diagnosis in vogue. Histopathology 1997;31:568-76.
- Suster S, Nascimento AG, Miettinen M, et al. Solitary fibrous tumors of soft tissue. A clinicopathologic and immunohistochemical study of 12 cases. Am J Surg Pathol 1995;19:1257-66.
- Hasegawa T, Hirose T, Seki K, et al. Solitary fibrous tumor of the soft tissue. An immunohistochemical and ultrastructural study. Am J Clin Pathol 1996;106:325-31.
- Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol 1998;22:1501-11.
- Morimitsu Y, Nakajima M, Hisaoka M, et al. Extrapleural solitary fibrous tumor: clinicopathologic study of 17 cases and molecular analysis of the p53 pathway. APMIS 2000;108:617-25.
- van de Rijn M, Lombard CM, Rouse RV. Expression of CD34 by solitary fibrous tumors of the pleura, mediastinum, and lung. Am J Surg Pathol 1994;18:814-20.
- Chilosi M, Facchettti F, Dei Tos AP, et al. bcl-2 expression in pleural and extrapleural solitary fibrous tumours. J Pathol 1997;181:362-7.
- Suster S, Fisher C, Moran CA. Expression of bcl-2 oncoprotein in benign and malignant spindle cell tumors of soft tissue, skin, serosal surfaces, and gastrointestinal tract. Am J Surg Pathol 1998;22:863-72.
- Dal Cin P, Pauwels P, Van Den Berghe H. Solitary fibrous tumour of the pleura with t(4;15)(q13;q26). Histopathology 1999;35:94-5.
- Donner LR, Silva MT, Dobin SM. Solitary fibrous tumor of the pleura: a cytogenetic study. Cancer Genet Cytogenet 1999;111:169-71.
- Havlik DM, Farnath DA, Bocklage T. Solitary fibrous tumor of the orbit with a t(9;22)(q31;p13). Arch Pathol Lab Med 2000;124:756-8.
- de Saint Aubain Somerhausen N, Rubin BP, Fletcher CD. Myxoid solitary fibrous tumor: a study of seven cases with emphasis on differential diagnosis. Mod Pathol 1999;12:463-71.
- Tani Y, Tateno T, Izumiyama H, et al. Defective expression of prohormone convertase 4 and enhanced expression of insulin-like growth factor II by pleural solitary fibrous tumor causing hypoglycemia. Endocr J 2008;55:905-11.
- Duguay SJ, Jin Y, Stein J, et al. Post-translational processing of the insulin-like growth factor-2 precursor. Analysis of O-glycosylation and endoproteolysis. J Biol Chem 1998;273:18443-51.
- Qiu Q, Basak A, Mbikay M, et al. Role of pro-IGF-II processing by proprotein convertase 4 in human placental development. Proc Natl Acad Sci USA 2005;102:11047-52.
- Frystyk J, Skjaerbaek C, Zapf J, et al. Increased levels of circulating free insulin-like growth factors in patients with non-islet cell tumour hypoglycaemia. Diabetologia 1998;41:589-94.
- Bond JJ, Meka S, Baxter RC. Binding characteristics of pro-insulin-like growth factor-II from cancer patients: binary and ternary complex formation with IGF binding proteins-1 to -6. J Endocrinol 2000;165:253-60.
- England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol 1989;13:640-58.
- de Perrot M, Kurt AM, Robert JH, et al. Clinical behavior of solitary fibrous tumors of the pleura. Ann Thorac Surg 1999;67:1456-9.


