Current challenges in interventional mitral valve treatment
Introduction
Mitral regurgitation (MR) is the most prevalent valve disease in the western population and it is associated with reduced life expectancy and increased risk of heart failure (1).
Surgical intervention is indicated in presence of severe MR and symptoms of heart failure, or when patients present with reduced left ventricle (LV) function, pulmonary hypertension, or atrial fibrillation (2). However, up to 50% of the symptomatic patients with severe MR are not referred to surgery due to an expected high surgical risk (3). Transcatheter mitral valve therapies have emerged as an alternative option. Multiple technologies and diversified approaches are today under clinical study or in development. They can be categorized based on the anatomical and pathophysiological addressed target: leaflet and chordal repair procedures, indirect and direct annuloplasty, LV remodeling devices and valve replacement devices (4).
As multiple technologies and different approaches will become available in the field of mitral valve interventions, different challenges are emerging, both patient (clinical challenges) and procedure-related (technical challenges). This review will briefly explore the current open challenges in the evolving fields of interventional mitral valve treatment.
Clinical challenges
Increased complexity of the patients: the importance of a “global” patient selection
Clinical, ethical and economical implications
The increased complexity of the patients is strictly related to the increased expectancy of life and with the concept of “active ageing”. The prevalence of heart valve disease increases with age, with the predominance of degenerative etiology, as shown by the Euro Heart Survey on valvular heart disease (5). More than 10% of patients aged 75 years and more, requiring hospitalization, have significant MR (3). Many elderly patients are considered non-surgical candidates because of associated copathologies and high surgical risk.
In particular, in patients 80 years and older with severe MR, surgical treatment is performed in Europe only in 15%, as compared to 60% in patients aged 70 years and younger (3). With the availability of less invasive interventional mitral therapies, the clinical issue of the evaluation of the balance between utility vs. futility of any invasive treatment for each patient is raising, given the possibility of no improvement in the quality of life and no gaining independence back, even if the intervention is successful and the patient survives. Assessing the delicate point in which the clinical benefit is overpassed by the poor value or futility of the procedure is the biggest clinical challenge of physicians in this context. Multidisciplinary evaluation is mandatory to define the most appropriate therapy for each patient, taking into account the clinical status, the expected surgical risk and the anatomy of the mitral lesion (6).
Moreover, ethical implications should be taken into account. The therapeutic decision in this context should be reached also by informing the patient and the family thoroughly, taking into account their perspective. Concomitantly, the economic burden in high-risk elderly patients has crucial implications and should be considered before proceeding, due to reduced expectancy of life.
From a palliative to a prognostic approach?
Improved safety and efficacy, need for experienced operators specifically trained, combined approach in selected cases
The clinical target of interventional mitral valve treatment will probably move in the next future from a palliative towards a prognostic approach. Clinical experience with mitral valve intervention (both surgical and transcatheter) clearly demonstrated that early timing is crucial to accomplish substantial prognostic benefit (7-9): early repair can restore expectancy of life in degenerative MR (DMR) patients and induce reverse remodelling in functional MR (FMR). On the contrary, in patients treated in a too advanced phase of the disease, transcatheter mitral procedures may be unable to influence the prognosis (10,11). The safety of the procedure is fundamental to justify any early indications and probably plays a predominant role over efficacy in this specific context. Proven that transcatheter mitral valve therapies are relatively safe even in high-risk patients (12,13), the question of “how less efficacious is efficacious enough” patients is crucial to expand indications in lower risk patients.
The definition of a specific and shared educational training program of an interventional mitral valve operator (including both surgical and interventional skills) will represent a major challenge for the cardiovascular community in the next years.
Reproducibility of the procedures and experience of the operators play a fundamental role. In particular, every operator should become confident with different transcatheter devices in order to select the most appropriate approach for each patient and for different mitral anatomies. In selected cases, also a combination of different device should be taken in consideration in order to tailor the approach and to optimize the outcomes (i.e., leaflet repair and transcatheter annuloplasty in the same patient).
Technical challenges
New technologies and new approaches
Efficacy, reproducibility, versatility and technical complexity
Different anatomical lesions and aetiologies may underlie MR. Therefore, a series of different technologies and devices should be available. They can be categorized based on the anatomical and pathophysiological addressed target: leaflet and chordal repair procedures, indirect and direct annuloplasty and LV remodeling devices. Operators dedicated to transcatheter mitral interventions should become expert in using more than one device, in order to improve the outcomes and to increase the number of the patients potentially eligible for interventional mitral treatment.
Currently, MitraClip (Abbott Vascular, USA) has been a winning device mainly because it is versatile, and it can be used both in patients with FMR and DMR. Clinical and anatomical efficacy have been reported even in high-risk or inoperable patients with both functional and degenerative aetiologies (about 80% of the patients in NYHA class I-II 1 year after the procedure; about 80% of the patients with MR ≤2+ at 1 year) (12,14). MitraClip is CE Marked since 2008 and it also obtained FDA approval in 2013 for symptomatic patients with severe DMR at too high risk for surgery. About 20,000 patients have been worldwide treated up today.
However, MitraClip implantation is a procedure very different from other interventions.
Beyond the transseptal puncture, the procedure is more a robotic “surgical like” endovascular procedure, with free-floating navigation in a three-dimension (3D) space. Advanced 3D echocardiographic guidance is fundamental to obtain good results.
From a technical standpoint, most repair technologies on the horizon require advanced and specific skills.
Some technologies for transcatheter mitral repair are easier to perform, such as coronary sinus annuloplasty (indirect annuloplasty), which is based on the anatomical proximity of the coronary sinus to the posterior mitral annulus. This approach is particularly attractive because the cannulation of the coronary sinus is an easy and reproducible venous access technique. Initial results with this approach have not been satisfactory, mainly due to suboptimal efficacy and the risk of delayed complications (including coronary occlusion).
The Carillon Mitral Contour System (Cardiac Dimension, Inc., Kirkland, Washington) is the only technology still using this approach. It has recently obtained CE mark. The TITAN trial, which evaluated the clinical impact of Carillon Mitral in HF patients with at least moderate FMR, demonstrated significant reductions in FMR and in LV volumes. Functional status (including walking test performance) markedly improved in the implanted patients (15).
Although clinical benefits have been observed, this approach has a restricted applicability in the real world, mainly due to its limited efficacy (due to the fact that coronary sinus and mitral annulus are not coplanar) and to the risk of coronary artery compression and device dislocation.
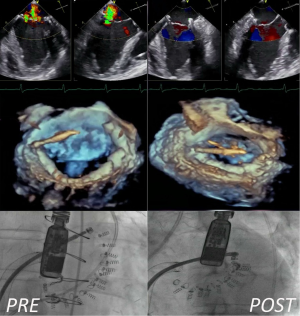
Recently direct annuloplasty is becoming available. Direct mitral valve annuloplasty repair is a very promising approach for transcatheter mitral valve repair, since it closely reproduces a gold-standard surgical method. The Cardioband (ValtechCardio Inc, Israel) is the closest transcatheter device to a surgical prosthetic ring. Delivered through a transseptal access, the implant of a surgical-like adjustable Dacron band is performed on the atrial side of the mitral annulus, from trigone to trigone, by means of multiple anchors (16,17). Ongoing CE trial is enrolling high-risk patients with FMR and initial clinical data are promising, showing significant reduction of MR and improved functional status after the procedure.
Promising initial clinical results have been showed also with the Mitralign device (Mitralign, Inc, USA), which performs selective plication of the mitral annulus by deploying couples of trans-annular pledgets in the annulus (18). Patient enrollment in the CE mark trial is completed, but the device is still not available for clinical use.
The Accucinch System (Guided Delivery Systems, USA) is another direct annuloplasty device that uses the retrograde transventricular approach. The Accucinch System also causes remodeling of the basal portion of the LV, promoting papillary muscle approximation, and is unique in this respect. Limited clinical data are available.
Today, the main concern about mitral direct annuloplasty repair is that these procedures are technically very challenging and require advanced integrated imaging guidance (Figure 1): these aspects could limit their rapid and effective adoption in the real world.
Beyond indirect and direct annuloplasty, other methods have been attempted to remodel the mitral annulus, including external compression of atrio-ventricular groove, implant of cinching devices and application of radiofrequency or ultrasound energy sources to shrink the annular collagen (19-22). Reproducibility, efficacy and safety of these appealing technologies needs still to be proved because they are based on novel concepts, without a validated and reproducible surgical or preclinical background.
The transcatheter repair armamentarium includes also artificial chordae implantation, which are anchored to the LV apex to restore mitral valve competency (NeoChord, Inc., Eden Prairie, Minnesota) (23). This procedure is performed through a transapical, off-pump, beating heart approach. It has been used successfully in several patients, with the evidence of a learning curve. Long-term durability of this approach remains an issue.
Different ventricular or atrial remodelling devices complete the wide spectrum of repair technologies, but limited clinical data are today available for these approaches.
Transcatheter mitral valve replacement is an emerging clinical field. Percutaneous mitral valve replacement has proved to be feasible as valve-in-valve and valve-in-ring procedure in patients at high surgical risk (24). Moreover, the feasibility of the implant of a percutaneous balloon-expandable aortic prosthesis in a native severely calcified mitral valve has been reported (25). The feasibility of transcatheter mitral valve implantation in native non-calcified valves has been recently reported in very high-risk patients, mainly with FMR (26).
When mitral replacement technologies will become clinically available, it will be even more difficult to identify the ideal therapy for the individual patient. The valves that have been today implanted in native mitral position are self-expandable prosthesis, mainly delivered through the transapical route (less than 20 cases overall have been reported with different devices). Mitral replacement may offer some theoretical advantages, as reproducibility and applicability to majority of patients, with a high predictable and less technically demanding procedure. However, transcatheter mitral valve repair, although more complex and technically challenging, is more physiological and therefore associated with a superior safety profile, as compared to replacement, since it does not involve a heterologous tissue implant and does not require anticoagulation. The overall balance between advantages and disadvantages of the two approaches introduces the need for patient-specific tailored approach. In the future, transcatheter mitral repair and replacement will probably have a complementary role rather than competitive. Specific algorithms with specific indications will be required to guide the optimal treatment for each patient.
Integrated imaging
Pre-procedural screening, patient selection and intra-procedural guidance
Given the previously mentioned complex pathophysiology of mitral valve disease, the need of a full-comprehensive assessment of the valvular anatomy, the adjacent cardiac and extra-cardiac structures and the peripheral accesses must be performed in the pre-operative evaluation. Hence, a multi-disciplinary formation with a vast understanding in both sonographic and tomographic images is already become a fundamental skill of the modern interventional mitral valve operator.
With minor differences that may be detected if looking at the single centre case, all patients referred for percutaneous operation on mitral valve are routinely enrolled in preoperative protocols, which generally include a chest X-ray, a coronarography, a combined trans-thoracic echocardiography (TTE) and trans-esophageal echocardiography (TEE). In fact, a preoperative extensive study of the mechanism of regurgitation and the valvular anatomy, inclusive of details on the localization of the regurgitant orifice, the presence of anatomical features that are associated to poor implant success (e.g., short grasping area, presence of clefts) or higher operative risk (e.g., extensive calcification of the annuls), is mandatory for the decision making. The integration of 3D reconstructions on TEE has been tested and reported as a powerful tool on preoperative screening for MR (27). Nevertheless, sub-optimal sonographic windows, heavy calcification or contraindication to TEE may impair this fundamental diagnostic step.
In order to avoid these complications and to simplify the reading of the images by the operators, advances in imaging techniques such as multidetector row computed tomography (MDCT), and cardiac magnetic resonance (CMR) may be helpful especially when integrated in a multimodality approach (28).
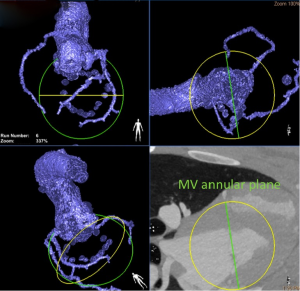
Multiphase retrospectively gated MDCT with its high degree of image quality and short acquisition time is typically required for assessment of valvular structure (29) good grade of correlation with TTE and CMR (30). Geometrical analyses of valvular and sub-valvular apparatus (e.g., annulus deformation, papillary muscles displacement and changing in leaflets angles) and of the relationship between mitral valve and other adjacent structures (e.g., coronary-sinus dimension, aorto-mitral angle, aorto-trigonal distance and distance from left atrial appendage) may also be accomplished by MDCT (31). This is the reason why, in preclinical context, MDCT is the method of choice in the preoperative screening for direct annuloplasty devices and this statement is also extendible in clinical practice (32). Interestingly, MDCT can be used to optimize operative fluoroscopic viewing angles of left-sided heart structures (33). Other advantages of these imaging modalities on the sonographic techniques are the output of useful 3D datasets with high-resolution submillimeter isotropic voxels, the concomitant evaluation of the coronary arteries and other cardiac and extracardiac structures of interest.
Less clear is the role of preoperative CMR in patients who are undergoing a percutaneous MV intervention. With regards to patients treated with MitraClip, CMR has been proven to be a useful tool in both preoperative and follow-up phase providing clinicians with a quantitative assessment of MR severity, insights into the mechanism of regurgitation, and an assessment of the consequences of the regurgitant lesion on LV volumes and systolic function (34).
Once again, MDCT can be used in patient populations that are unable to undergo CMR studies, such as patients with claustrophobia, pacemakers, and implantable defibrillators.
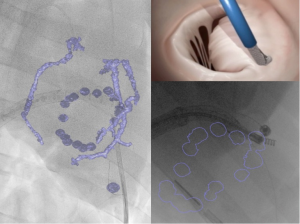
Intraprocedural imaging for percutaneous MV interventions represents the actual real challenge for imaging. The complexity of manoeuvring through TEE and fluoroscopy guidance steerable catheters delivering clips, artificial valves or anchors in the narrow 3D space of the left heart chambers could be often addressed as the reason for a suboptimal implantation, long operative and fluoroscopic time and intraoperative complications. Given the importance for the navigation of the device on the beating heart and the expected optimal control on MR, the huge amount of hybrid imaging systems, consisting of fluoroscopy and echocardiography, are increasingly selected for intraoperative support.
Intracardiac echocardiography (ICE) is an emerging modality commonly used only in preclinical phase. Even though the attractive promise of removing sedation or general anaesthesia associated with TEE, this technique allows the inspection from only one projection, with different degree of freedom.
As mentioned above, specific preoperative MDCT analyses may be used to simplify the deployment of the valve prosthesis by pre-selecting optimal fluoroscopic viewing angles (33).
A new hybrid imaging system, consisting of an overlay 3D-MDCT data onto the real-time procedural fluoroscopy (HeartNavigator software, Philips Healthcare, Best, Netherlands), was also found to be feasible and to provide a simplified procedural guidance for complex structural cardiac intervention (Figures 2,3).
Conclusions
As multiple technologies and different approaches will become available in the field of mitral valve interventions, different clinical and technical challenges are emerging, facing with the rapid increase of the number of potential patients who are not amenable for conventional surgery. New and reproducible procedures, advanced integrated imaging modalities, specific hybrid training programs and a modern heart-team approach are the keys to overcome all the challenges of interventional mitral valve treatments.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875-83. [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [PubMed]
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358-65. [PubMed]
- Maisano F, Buzzatti N, Taramasso M, et al. Mitral transcatheter technologies. Rambam Maimonides Med J 2013;4:e0015. [PubMed]
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [PubMed]
- Taramasso M, Gaemperli O, Maisano F. Treatment of degenerative mitral regurgitation in elderly patients. Nat Rev Cardiol 2015;12:177-83. [PubMed]
- Detaint D, Sundt TM, Nkomo VT, et al. Surgical correction of mitral regurgitation in the elderly: outcomes and recent improvements. Circulation 2006;114:265-72. [PubMed]
- De Bonis M, Lapenna E, Verzini A, et al. Recurrence of mitral regurgitation parallels the absence of left ventricular reverse remodeling after mitral repair in advanced dilated cardiomyopathy. Ann Thorac Surg 2008;85:932-9. [PubMed]
- Enriquez-Sarano M, Sundt TM 3rd. Early surgery is recommended for mitral regurgitation. Circulation 2010;121:804-11; discussion 812. [PubMed]
- Neuss M, Schau T, Schoepp M, et al. Patient selection criteria and midterm clinical outcome for MitraClip therapy in patients with severe mitral regurgitation and severe congestive heart failure. Eur J Heart Fail 2013;15:786-95. [PubMed]
- Taramasso M, Maisano F, Latib A, et al. Clinical outcomes of MitraClip for the treatment of functional mitral regurgitation. EuroIntervention 2014;10:746-52. [PubMed]
- Maisano F, Franzen O, Baldus S, et al. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol 2013;62:1052-61. [PubMed]
- Nickenig G, Estevez-Loureiro R, Franzen O, et al. Percutaneous mitral valve edge-to-edge repair: in-hospital results and 1-year follow-up of 628 patients of the 2011-2012 Pilot European Sentinel Registry. J Am Coll Cardiol 2014;64:875-84. [PubMed]
- Glower DD, Kar S, Trento A, et al. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: results of the EVEREST II study. J Am Coll Cardiol 2014;64:172-81. [PubMed]
- Siminiak T, Wu JC, Haude M, et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: results of the TITAN Trial. Eur J Heart Fail 2012;14:931-8. [PubMed]
- Maisano F, La Canna G, Latib A, et al. First-in-man transseptal implantation of a "surgical-like" mitral valve annuloplasty device for functional mitral regurgitation. JACC Cardiovasc Interv 2014;7:1326-8. [PubMed]
- Maisano F, Vanermen H, Seeburger J, et al. Direct access transcatheter mitral annuloplasty with a sutureless and adjustable device: preclinical experience. Eur J Cardiothorac Surg 2012;42:524-9. [PubMed]
- Siminiak T, Dankowski R, Baszko A, et al. Percutaneous direct mitral annuloplasty using the Mitralign Bident system: description of the method and a case report. Kardiol Pol 2013;71:1287-92. [PubMed]
- Raman J, Jagannathan R, Chandrashekar P, et al. Can we repair the mitral valve from outside the heart? A novel extra-cardiac approach to functional mitral regurgitation. Heart Lung Circ 2011;20:157-62. [PubMed]
- Palacios IF, Condado JA, Brandi S, et al. Safety and feasibility of acute percutaneous septal sinus shortening: first-in-human experience. Catheter Cardiovasc Interv 2007;69:513-8. [PubMed]
- Heuser RR, Witzel T, Dickens D, et al. Percutaneous treatment for mitral regurgitation: the QuantumCor system. J Interv Cardiol 2008;21:178-82. [PubMed]
- Jilaihawi H, Virmani R, Nakagawa H, et al. Mitral annular reduction with subablative therapeutic ultrasound: pre-clinical evaluation of the ReCor device. EuroIntervention 2010;6:54-62. [PubMed]
- Seeburger J, Rinaldi M, Nielsen SL, et al. Off-pump transapical implantation of artificial neo-chordae to correct mitral regurgitation: the TACT Trial (Transapical Artificial Chordae Tendinae) proof of concept. J Am Coll Cardiol 2014;63:914-9. [PubMed]
- Cheung A, Webb JG, Barbanti M, et al. 5-year experience with transcatheter transapical mitral valve-in-valve implantation for bioprosthetic valve dysfunction. J Am Coll Cardiol 2013;61:1759-66. [PubMed]
- Himbert D, Bouleti C, Iung B, et al. Transcatheter valve replacement in patients with severe mitral valve disease and annular calcification. J Am Coll Cardiol 2014;64:2557-8. [PubMed]
- Cheung A, Webb J, Verheye S, et al. Short-term results of transapical transcatheter mitral valve implantation for mitral regurgitation. J Am Coll Cardiol 2014;64:1814-9. [PubMed]
- Debonnaire P, Delgado V, Bax JJ, et al. Tools & Techniques - Clinical: 3D transoesophageal echocardiography for selecting and guiding in percutaneous mitral valve repair using MitraClip. EuroIntervention 2014;10:884-6. [PubMed]
- Delgado V, Kapadia S, Marsan NA, et al. Multimodality imaging before, during, and after percutaneous mitral valve repair. Heart 2011;97:1704-14. [PubMed]
- Buttan AK, Yang EH, Budoff MJ, et al. Evaluation of valvular disease by cardiac computed tomography assessment. J Cardiovasc Comput Tomogr 2012;6:381-92. [PubMed]
- Guo YK, Yang ZG, Ning G, et al. Isolated mitral regurgitation: quantitative assessment with 64-section multidetector CT--comparison with MR imaging and echocardiography. Radiology 2009;252:369-76. [PubMed]
- Delgado V, Tops LF, Schuijf JD, et al. Assessment of mitral valve anatomy and geometry with multislice computed tomography. JACC Cardiovasc Imaging 2009;2:556-65. [PubMed]
- Sündermann SH, Gordic S, Manka R, et al. Computed tomography for planning and postoperative imaging of transvenous mitral annuloplasty: first experience in an animal model. Int J Cardiovasc Imaging 2015;31:135-42. [PubMed]
- Thériault-Lauzier P, Andalib A, Martucci G, et al. Fluoroscopic anatomy of left-sided heart structures for transcatheter interventions: insight from multislice computed tomography. JACC Cardiovasc Interv 2014;7:947-57. [PubMed]
- Krumm P, Zuern CS, Wurster TH, et al. Cardiac magnetic resonance imaging in patients undergoing percutaneous mitral valve repair with the MitraClip system. Clin Res Cardiol 2014;103:397-404. [PubMed]


