Systematic review and meta-analysis of the efficacy and safety of therapy with linezolid containing regimens in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis
Introduction
Tuberculosis (TB) has attracted increasing public health attention due to the alarming rates of multidrug-resistant TB (MDR-TB, defined as resistance to at least isoniazid and rifampicin) and extensively drug-resistant TB (XDR-TB, defined as MDR-TB with additional bacillary resistance to any fluoroquinolone and at least one second-line injectable anti-TB drug) (1,2). MDR accounts for about 3.6% of new TB patients worldwide, accounting for an estimated 450,000 new cases in 2012. By September 2013, 92 countries had reported at least one case of XDR-TB. Among the developing countries, India and China has the two highest prevalence of TB in the world and troublingly high rates of MDR and XDR-TB (3,4). Furthermore, the treatment of MDR-TB requires a longer duration, is considerably more complicated, expensive and toxic. The longer treatment course of MDR-TB results in poor treatment outcome, leading to the emergence of XDR-TB. XDR-TB treatment is much more difficult and costly and will stress national health budgets even more than MDR-TB treatment (5-8).
Recently a number of promising novel strategies for the management of drug-resistant TB have also been explored, including treatment with linezolid, fluoroquinolones, high-dose isoniazid and phenothiazines (9). A linezolid-containing regimen for drug-resistant TB (DR-TB) is one of the off-label uses of linezolid that has not yet been approved by the licensing agencies. Excellent activity against drug-resistant Mycobacterium TB was in fact discovered at the early stage of in vitro studies of linezolid. However, in contrast with other protein synthesis inhibitors, linezolid’s mechanism of action involves inhibition of protein synthesis at its early stages (10-13).
So far, the efficacy of linezolid for the treatment of TB has never been documented by clinical trials. Current treatment for MDR-TB lasts 20 months at least (14). The currently approved time for licensed treatments using linezolid is up to 28 days; however, this is an insufficient length of time for treatment of DR-TB. Thus the potential effectiveness of a linezolid-containing regimen for DR-TB must be clinically assessed against the dangers of drug toxicity caused by extended use (10). However, since the outcome of patients with DR-TB is generally poor (15), other treatment options need to be considered. Linezolid has excellent activity against DR mycobacteria and its effectiveness and safety have been evaluated in several small studies, including a randomized controlled trial (RCT) with a small number of patients and a suboptimal study design. In the present systematic review and meta-analysis we sought to pool the clinical evidence regarding the effectiveness and safety of linezolid for the treatment of DR-TB.
Methods
Search strategy
We searched the Cochrane Library, PubMed, Embase, China National Knowledge Infrastructure (CNKI) and Science Citation Index Expanded (SCI) database from January 2000 to May 2014, using combinations of the key words: “linezolid”, “MDR-TB”, “XDR-TB”, “multidrug-resistant tuberculosis” and “extensively drug-resistant tuberculosis”. In addition, the references of the chosen articles and relevant review papers were hand-searched and reviewed. The review was not restricted by language.
Selection strategy
Two investigators (X Zhang and R Qin) independently searched the literature and examined relevant studies for further assessment of data on effectiveness and safety of linezolid for the treatment of patients with DR-TB. We included peer-reviewed reports of studies from which the treatment outcomes of the patients could be clearly extracted. A number of criteria were required for inclusion in our analysis: (I) confirmation that patients had MDR-TB by drug-susceptibility testing (DST) of M. tuberculosis cultures; (II) any individualized treatment could be given to patients, provided linezolid was included in a regimen after the study enrolment; (III) detailed treatment outcomes and follow-up data or definitions of treatment success similar to that outlined by World Health Organization (WHO) were provided; (IV) the proportion of patients with pulmonary TB was not less than 90%. Exclusion criteria of studies for our analysis were: (I) experimental trials and trials focused solely on pharmacokinetic or pharmacodynamic variables; (II) animal studies; (III) reports that appeared in conference abstracts with no further information obtainable from personal correspondence with the authors.
Data extraction
Data were extracted independently by two investigators (R Qin and X Zhang), and differences were resolved by discussion with a third investigator (R Wang). The corresponding authors of selected papers were contacted to obtain any missing data, including those for the assessment of the effectiveness, safety and tolerability of linezolid-containing treatment regimens. Basic study information, including the first author, publication year, country in which the study was conducted, clinical center, study period and study design, the number of MDR- and XDR-TB patients, the proportion of XDR-TB patients, the rates of infection related to gender, average age and the history of previous anti-TB treatment was extracted.
For evaluation of linezolid effectiveness, safety and tolerability, the following variables were collected: the dosage of linezolid, the final treatment outcome, adverse events, and the rate of sputum culture conversion and the duration of exposure to the linezolid-containing regimen.
Definitions
According to the recommendations of the WHO, we defined the final treatment outcome as either favorable (cured and treatment completed) or unfavorable (died, treatment failed) (16). Safety and tolerability end-points are classified as major and minor adverse events. A major adverse event was defined as any adverse reaction that brought about temporary or permanent discontinuation of linezolid, whereas a minor adverse event required only dose adjustment and/or addition of concomitant treatment (17).
Qualitative assessment
This systematic review and meta-analysis of studies on the use of linezolid for DR-TB was performed according to guidelines established by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group (18). Discrepancies for the study selection and data extraction from the included studies were resolved by consensus (X Zhang and R Qin).
Data analysis
We pooled the proportions of patients who had a favorable outcome (cured and treatment completed), sputum culture conversion, or failed and died across the selected studies for a meta-analysis, using available detailed data from patients. The 95% score interval method was used to calculate the 95% CI. Random-effects models were applied for assigning weights according to the methods described by DerSimonian and Laird (19).
The heterogeneity of outcome within and between each group of studies were assessed using the Cochrane Q test (P value <0.1 denoting the presence of heterogeneity) and the I2 statistic in forest plots described by Higgins et al. (20,21). The I2 statistic estimates the percent of observed between-study variability due to heterogeneity rather than to chance and ranges from 0 to 100 percent (values of 25%, 50% and 75% were considered representing low, medium and high heterogeneity respectively) (22). A value of 0% indicates no observed heterogeneity while 100% indicates significant heterogeneity. For this review we determined that I2 values above 75 percent were indicative of significant heterogeneity. This heterogeneity was further explored through subgroup analyses and meta-regression. Subgroup analyses were performed based on the potential clinical factors when such were available (e.g., initial daily dose of linezolid ≤600 or >600 mg). A univariate approach was employed to assess the causes of heterogeneity among the selected studies. We tested the significance of these coefficients by Student’s t-test and reported the P value for significance (23). To avoid false negative results due to the small number of studies entered in the regression analysis, a two-tailed 0.10 P value was considered as cutoff for statistical heterogeneity in a meta-regression.
To clearly define data from different statistical methods, when 95% CI is presented a meta-analysis was done; otherwise the data was added. Statistical procedures were performed using the STATA version 10 and R 2.15.2 (24).
Finally, the small study bias was appraised by graphical inspection of funnel plots and Egger test.
Results
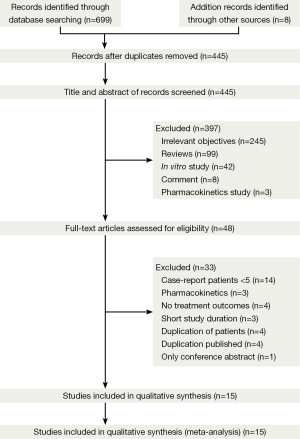
Study selection
Of the 414 publications that we initially identified as dealing with MDR- and XDR-TB, 370 were excluded because they were reviews, commentaries, in vitro studies, or were irrelevant to our research objectives (Figure 1). We performed a full text review on 44 articles, 15 of which met our specified inclusion and exclusion criteria (17,25-38).
Characteristics of studies and assessment of study qualities
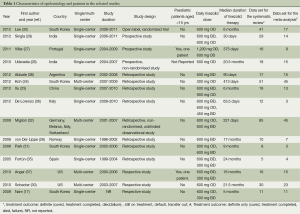
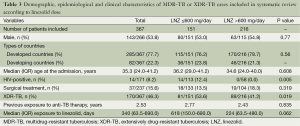
Table 1 summarizes the basic characteristics of the 15 studies included in the analysis. Most (46.7%) were conducted in Asian countries, such as South Korea, India and China, or in Europe (33.3%). The majority of the studies (73.3%) were retrospective and observational in design; only one was a RCT (33). All 15 studies were published in English. Of the 15 studies, four were multicenter studies (29,32,34,37).
Full table
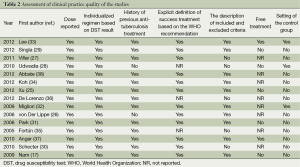
As shown in the assessment of clinical practice quality (Table 2), the study by Lee et al. was a RCT (33). Two studies provided free treatment for patients (33,37). However, the quality of the treatment given to patients was judged to be good, because dosages in all the studies were recorded clearly and all patients were prescribed individualized treatment according to the DST result. The majority of studies (87%) had recorded the history of previous anti-TB treatments.
Full table
Characteristics of patients included
In the publications studied, 367 patients met the definition for infection with MDR- or XDR-TB, and 239 patients had evaluable treatment outcomes (Table 1). None of the authors contacted via e-mail provided the additional requested data. More than half the patients were male with a median age of 35.3 (range, 24-41) years. Nearly 9% of patients were HIV-positive. The mean number of drugs included in the MDR- and XDR-TB treatment ranged from 5 to 11, but the specific antibiotics or changes in the antibiotic regimens other than linezolid were not provided in the majority of the studies. The mean percentage of patients undergoing surgery was 18.1% (range, 0-63.6%). XDR-TB accounted for 46.3% of the included cases.
In the comparative analysis according to linezolid dose (Table 3), with the exception of the proportions of XDR-TB and HIV-positive patients, no statistically significant differences were detected in the characteristics of those who were treated with a daily linezolid-containing regimen of ≤600 mg and those with a daily dose of >600 mg. The group with a daily dose of ≤600 mg had a higher proportion of HIV-positive and XDR-TB patients.
Full table
Effectiveness of linezolid-containing regimens
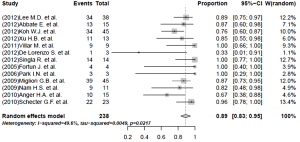
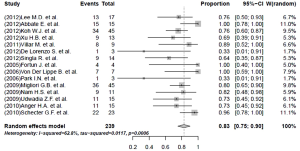
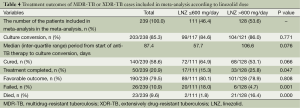
The pooled rate of culture conversion of the patients who had evaluable treatment outcomes was 89% (95% CI, 83-95%; I2=49.6%) (Figure 2). Of the 239 patients who had evaluable treatment outcomes, 190 MDR-TB patients had favorable outcomes. The weighted proportion of favorable outcome was 83% (95% CI, 75-90%; I2=62.8%, Figure 3). Death and treatment failure were observed in 9.6% and 10.9% of the enrolled subjects, respectively.
Patients receiving a lower dosage of linezolid (≤600 mg) had a lower death rate (1.8% vs. 16.4%, P value <0.05) but a higher percentage of failure (18.0% vs. 4.7%, P value =0.001), than the group receiving the higher dosage (>600 mg) (Table 4).
Full table
Safety and tolerability of linezolid-containing regimen
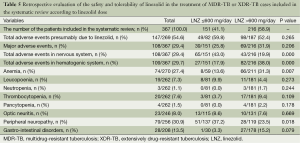
Nearly half the patients treated with a linezolid-containing regimen experienced adverse events that were attributable to linezolid (Table 5). Major adverse effects, which required linezolid discontinuation, were experienced by 35% (95% CI, 22-47%; I2=90.1%) of the patients (Figure 4). Peripheral neuropathy (31%, 95% CI, 19-42%; I2=81.7%) and anemia (25%, 95% CI, 15-34%; I2=76.6%) constituted the main adverse effects (Figures 5 and 6, respectively). Other linezolid-related adverse events with lower frequencies included gastro-intestinal disorders (28/208, 13.5%), optic neuritis (23/246, 8.0%), thrombocytopenia (20/262, 7.6%) and leucopenia (19/262, 7.3%) (Table 5).
Full table
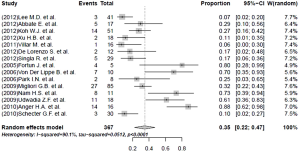
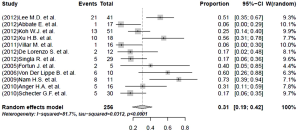
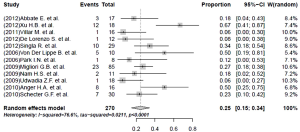
A higher incidence of adverse events in the hematopoetic system (38.0% vs. 17.9%, P value <0.05) and a lower frequency of adverse events in nervous system (19.9% vs. 43.0%, P value <0.05) attributed to linezolid treatment were detected in the subgroup with a linezolid dosage >600 mg/day. More specifically, a statistically significantly higher risk of anemia (31.3% vs. 13.6%, P value =0.007) and a lower proportion of peripheral neuropathy (23.5% vs. 37.2%, P value=0.018) was found in the group with a linezolid dose of >600 mg/day, compared with the group taking a dose of ≤600 mg/day.
Heterogeneity analysis
Subgroup analyses on the effectiveness, safety and tolerability were performed based on the potential clinical factors between patients treated with a daily dose of linezolid ≤600 and >600 mg. No differences were found in the baseline characteristics of patients between the group treated with high dose or low dose of linezolid, except in the rates of XDR-TB and HIV, which were both slightly higher in the group receiving lower linezolid dose. This may have been one of the factors that caused high heterogeneity.
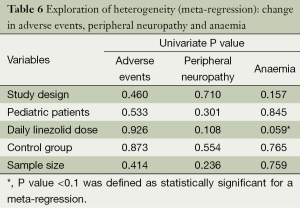
A high level of statistical heterogeneity was observed in the level of adverse events (I2=90.1%) (Figure 4), peripheral neuropathy (I2=81.7%) and anaemia (I2=76.6%) across studies. A meta-regression was introduced to explore the heterogeneity between studies. This evaluated the impact of study characteristics on the outcomes. The following variables for each study were assessed: (I) study design; (II) pediatric patients; (III) daily linezolid dose; (IV) control group; and (V) sample size. The results of the exploration of this heterogeneity are shown in Table 6. No significant differences were shown (Table 6).
Full table
Discussion
In this systematic review and meta-analysis we sought to evaluate the effectiveness and safety of linezolid as salvage treatment of patients with DR-TB. The pooled analysis showed that linezolid was associated with a promising favorable outcome in 83% of treated patients and sputum conversion in 89% of them. The findings of the analysis were strengthened by the fact that linezolid was added in the regimen after treatment with first or second line agents had failed. Compared with previous meta-analyses, the inclusion of more clinical studies and a larger sample size (approximately 70% more patients were included) in this analysis allowed a more precise assessment of data regarding the effectiveness and safety of linezolid-containing regimens (39,40). Finally, the addition of a small RCT added further accreditation to the analysis. In addition, we explored the possible origin of heterogeneity, an aspect not previously addressed.
Effectiveness
The pooled rate of culture conversion reached almost 90% (I2 <50%). The pooled proportion of the patients who experienced favorable outcomes was 83%, a figure higher than the treatment outcome from a recent meta-analysis of patients with DR-TB (67.99%; 95% CI, 58.00-78.99%) (39). This high level of treatment success may not be solely attributed to linezolid use but may be partly a reflection of the combination of drugs in these anti-TB regimens. All the patients included were receiving treatment with first- and second-line drugs for at least a month; data regarding specific antibiotics administered concurrently with linezolid were not available in the included studies. Furthermore, this might represent a form of publication bias. The WHO reports that the proportion of favorable outcome in patients TB was 87% in 2011. The respective proportion for patients with MDR-TB was 48% in 2010, while only approximately 30% of countries achieved a favorable outcome higher than 75% (2). Furthermore, the estimated mortality for MDR-TB was around 38%, while these studies reported a much lower mortality (approximately 10%). Thus, it is possible that data from settings where the effectiveness of linezolid was not that pronounced have not become publicly available. However, the RCT included in the analysis showed a sputum conversion of 87% at 6 months, suggesting that when linezolid is administered such a response may be feasible (33). In this RCT, patients were randomized to immediate or late prescription of linezolid and 4 months later were re-randomized to lower linezolid dose. Patients who received linezolid immediately were more likely to have culture conversion at 4 months than patients who received linezolid at two months (79% vs. 35%, P value =0.001).
There was no significant difference in favorable outcome between the group receiving daily linezolid doses of ≤600 or >600 mg. However, more patients completed treatment in daily linezolid dose >600 mg, while higher mortality and lower failure rate (P value <0.001) was found in the high dose group. We were unable to study why these differences were observed. We could assume that patients assigned to higher daily doses had more severe or unresponsive forms of TB because more patients in this group had an operation for their condition than in the lower dose group (although the difference was not significant). On the other hand, more patients in the lower dose group had HIV (although they were few in absolute numbers) and more patients had XDR TB. Data from RCTs regarding the optimal linezolid dose for DR-TB are not currently available.
The 35% incidence of major adverse effects requiring discontinuation or dose reduction of linezolid found in our analysis confirms the serious limitations of linezolid-containing treatments that were found in a previous works (40). In addition, a higher rate of toxicity in the hematopoietic system and a lower percentage of nervous toxicity (P value <0.01) were found in the group with higher linezolid dose. Furthermore, patients who received lower linezolid doses, received treatment for a longer period than those who were prescribed higher ones. Therefore, it seems that toxicity in hematopoietic system (myelosuppression) appeared to be more dose-dependent (41), while nervous system toxicity was duration-dependent (peripheral and optic neuropathy) (42,43). In accordance to the findings of this meta-analysis, the single available RCT reported that anemia developed mainly during the first 4 months, when patients were receiving mainly 600 mg daily, while neuropathy (optic and peripheral) developed mainly after 5 months of treatment, when most of patients were taking 300 mg daily (33).
The WHO suggested that the primary reasons for DR-TB are lower doses, and shorter periods of treatment or wrong antibiotics followed by transmission of DR-TB to close contacts. In addition, transmission due to diagnostic delays, and inadequate infection control programs have led to DR-TB becoming an increasing global health problem (2). Therefore, countries should be equipped to provide free access, quality medical care for M/XDR-TB among other interventions and try to improve diagnosis of latent TB infected individuals to achieve control and eventual elimination of TB (44,45). In addition, as one part of the care of patients with TB, therapeutic drug monitoring (TDM) may help the clinician make informed decisions regarding the timely adjustment of drug therapy (46). Especially, patients with M/XDR-TB, who are at risk of drug-drug interactions or have concurrent disease states, benefit more from TDM by reducing the dose of second line-drugs in order to reduce both adverse events and the cost of the drugs.
Limitations of our study
Our study was subject to several limitations. First of all, as the majority of the enrolled studies had some methodological limitations (e.g., non-randomized and retrospective studies), selection bias and publication bias could not be avoided in original studies. We acknowledged the risks of such biases by using a random-effect model which can be employed to control for fixed but unobserved heterogeneity (R>50%). Second, the combined results were influenced by some unpublished data in part of the clinical trials. For instance, two studies did not show the outcome of sputum culture conversion (25,27), and three studies did not record the total rate of adverse effects (27,28,34). Third, owing to the observational nature of the most of enrolled studies, the effectiveness of linezolid was not weighted for some confounding factors. The most pronounced of them was the lack of data regarding the specific antibiotics contained on the treatment regimens. (e.g., drug combination regimens).
Conclusions
In conclusion, the available data suggest that linezolid is a promising and viable option for treating DR-TB patients. However, doctors should carefully weigh up the benefits and drawbacks of using a linezolid-containing regimen according to the specific needs of individual patients. Although some studies recommend that dose reduction may sustain effectiveness and limit the adverse effect of myelosuppression (47), toxicity to the nervous system, triggered by prolonged use of linezolid, should always be considered. Meanwhile, patients being currently treated with linezolid need to be carefully monitored so that appropriate and timely intervention may be taken if adverse effects occur. Adequately powered RCTs can provide more valid conclusions regarding optimal doses and duration of linezolid treatment.
Acknowledgements
ME Falagas has participated in advisory boards of Astellas, Pfizerand, Bayer and has received lecture honoraria from Merck, Pfizer, AstraZeneca, Astellas, Cipla, Novartis, and Glenmark.
Disclosure: The authors declare no conflict of interest.
References
- Dalton T, Cegielski P, Akksilp S, et al. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet 2012;380:1406-17. [PubMed]
- World Health Organization. Global tuberculosis report 2013. World Health Organization, 2013.
- Dye C, Glaziou P, Floyd K, et al. Prospects for tuberculosis elimination. Annu Rev Public Health 2013;34:271-86. [PubMed]
- Yang C, Lei H, Wang D, et al. In vitro activity of linezolid against clinical isolates of Mycobacterium tuberculosis, including multidrug-resistant and extensively drug-resistant strains from Beijing, China. Jpn J Infect Dis 2012;65:240-2. [PubMed]
- Migliori GB, Sotgiu G, Gandhi NR, et al. Drug resistance beyond extensively drug-resistant tuberculosis: individual patient data meta-analysis. Eur Respir J 2013;42:169-79. [PubMed]
- Falzon D, Gandhi N, Migliori GB, et al. Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J 2013;42:156-68. [PubMed]
- Falzon D, Jaramillo E, Schünemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J 2011;38:516-28. [PubMed]
- Diel R, Vandeputte J, de Vries G, et al. Costs of tuberculosis disease in the European Union: a systematic analysis and cost calculation. Eur Respir J 2014;43:554-65. [PubMed]
- Field SK, Fisher D, Jarand JM, et al. New treatment options for multidrug-resistant tuberculosis. Ther Adv Respir Dis 2012;6:255-68. [PubMed]
- Vardakas KZ, Ntziora F, Falagas ME. Linezolid: effectiveness and safety for approved and off-label indications. Expert Opin Pharmacother 2007;8:2381-400. [PubMed]
- Alcalá L, Ruiz-Serrano MJ, Pérez-Fernández Turégano C, et al. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis that are susceptible or resistant to first-line antituberculous drugs. Antimicrob Agents Chemother 2003;47:416-7. [PubMed]
- Shinabarger D. Mechanism of action of the oxazolidinone antibacterial agents. Expert Opin Investig Drugs 1999;8:1195-202. [PubMed]
- Zurenko GE, Gibson JK, Shinabarger DL, et al. Oxazolidinones: a new class of antibacterials. Curr Opin Pharmacol 2001;1:470-6. [PubMed]
- World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis, 2011 Update. World Health Organization 2011.
- Ahuja SD, Ashkin D, Avendano M, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med 2012;9:e1001300. [PubMed]
- Laserson KF, Thorpe LE, Leimane V, et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2005;9:640-5. [PubMed]
- Nam HS, Koh WJ, Kwon OJ, et al. Daily half-dose linezolid for the treatment of intractable multidrug-resistant tuberculosis. Int J Antimicrob Agents 2009;33:92-3. [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [PubMed]
- Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101-29.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [PubMed]
- Jacobson KR, Tierney DB, Jeon CY, et al. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis 2010;51:6-14. [PubMed]
- Baschnagel AM, Yadav S, Marina O, et al. Toxicities and costs of placing prophylactic and reactive percutaneous gastrostomy tubes in patients with locally advanced head and neck cancers treated with chemoradiotherapy. Head Neck 2014;36:1155-61. [PubMed]
- Xu HB, Jiang RH, Li L, et al. Linezolid in the treatment of MDR-TB: a retrospective clinical study. Int J Tuberc Lung Dis 2012;16:358-63. [PubMed]
- von der Lippe B, Sandven P, Brubakk O. Efficacy and safety of linezolid in multidrug resistant tuberculosis (MDR-TB)--a report of ten cases. J Infect 2006;52:92-6. [PubMed]
- Villar M, Sotgiu G, D'Ambrosio L, et al. Linezolid safety, tolerability and efficacy to treat multidrug- and extensively drug-resistant tuberculosis. Eur Respir J 2011;38:730-3. [PubMed]
- Udwadia ZF, Sen T, Moharil G. Assessment of linezolid efficacy and safety in MDR- and XDR-TB: an Indian perspective. Eur Respir J 2010;35:936-8; author reply 938-40. [PubMed]
- Singla R, Caminero JA, Jaiswal A, et al. Linezolid: an effective, safe and cheap drug for patients failing multidrug-resistant tuberculosis treatment in India. Eur Respir J 2012;39:956-62. [PubMed]
- Schecter GF, Scott C, True L, et al. Linezolid in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis 2010;50:49-55. [PubMed]
- Park IN, Hong SB, Oh YM, et al. Efficacy and tolerability of daily-half dose linezolid in patients with intractable multidrug-resistant tuberculosis. J Antimicrob Chemother 2006;58:701-4. [PubMed]
- Migliori GB, Eker B, Richardson MD, et al. A retrospective TBNET assessment of linezolid safety, tolerability and efficacy in multidrug-resistant tuberculosis. Eur Respir J 2009;34:387-93. [PubMed]
- Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med 2012;367:1508-18. [PubMed]
- Koh WJ, Kang YR, Jeon K, et al. Daily 300 mg dose of linezolid for multidrug-resistant and extensively drug-resistant tuberculosis: updated analysis of 51 patients. J Antimicrob Chemother 2012;67:1503-7. [PubMed]
- Fortún J, Martín-Dávila P, Navas E, et al. Linezolid for the treatment of multidrug-resistant tuberculosis. J Antimicrob Chemother 2005;56:180-5. [PubMed]
- De Lorenzo S, Centis R, D'Ambrosio L, et al. On linezolid efficacy and tolerability. Eur Respir J 2012;39:770-2. [PubMed]
- Anger HA, Dworkin F, Sharma S, et al. Linezolid use for treatment of multidrug-resistant and extensively drug-resistant tuberculosis, New York City, 2000-06. J Antimicrob Chemother 2010;65:775-83. [PubMed]
- Abbate E, Vescovo M, Natiello M, et al. Successful alternative treatment of extensively drug-resistant tuberculosis in Argentina with a combination of linezolid, moxifloxacin and thioridazine. J Antimicrob Chemother 2012;67:473-7. [PubMed]
- Cox H, Ford N. Linezolid for the treatment of complicated drug-resistant tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis 2012;16:447-54. [PubMed]
- Sotgiu G, Centis R, D'Ambrosio L, et al. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur Respir J 2012;40:1430-42. [PubMed]
- Abena PA, Mathieux VG, Scheiff JM, et al. Linezolid and reversible myelosuppression. JAMA 2001;286:1973; author reply 1974.
- Bressler AM, Zimmer SM, Gilmore JL, et al. Peripheral neuropathy associated with prolonged use of linezolid. Lancet Infect Dis 2004;4:528-31. [PubMed]
- Rucker JC, Hamilton SR, Bardenstein D, et al. Linezolid-associated toxic optic neuropathy. Neurology 2006;66:595-8. [PubMed]
- Diel R, Loddenkemper R, Zellweger JP, et al. Old ideas to innovate tuberculosis control: preventive treatment to achieve elimination. Eur Respir J 2013;42:785-801. [PubMed]
- D'Ambrosio L, Dara M, Tadolini M, et al. Tuberculosis elimination: theory and practice in Europe. Eur Respir J 2014;43:1410-20. [PubMed]
- Srivastava S, Peloquin CA, Sotgiu G, et al. Therapeutic drug management: is it the future of multidrug-resistant tuberculosis treatment? Eur Respir J 2013;42:1449-53. [PubMed]
- Alffenaar JW, van Altena R, Harmelink IM, et al. Comparison of the pharmacokinetics of two dosage regimens of linezolid in multidrug-resistant and extensively drug-resistant tuberculosis patients. Clin Pharmacokinet 2010;49:559-65. [PubMed]