Addition to inhaled corticosteroids of leukotriene receptor antagonists versus theophylline for symptomatic asthma: a meta-analysis
Introduction
Asthma is a common chronic inflammatory disorder of the airways characterized by variable and recurring symptoms, reversible airflow obstruction and bronchial spasm (1). Anti-inflammatory therapy is the pharmacologic mainstay of asthma treatment and inhaled corticosteroids (ICSs) are currently the most effective anti-inflammatory medications in reducing asthma symptoms, improving the lung function, and reducing the airway inflammation in asthma.
When the asthma is poorly controlled by ICS monotherapy, other drugs such as long acting β2 agonists (LABAs), leukotriene receptor antagonists (LTRAs) and sustained release theophylline can be added. The recommended option is to combine a low dose of ICS with LABA (2). Addition of LABA leads to greater improvement in lung function, symptoms, and use of rescue β2 agonists, and to reduce the risk of exacerbations than increasing the dose of ICS (3). In addition, the approach of using a single inhaler containing ICS and LABA for both maintenance and reliever therapy (SMART) is reported to be superior in preventing exacerbations compared with conventional ICS-LABA combination (4). However, the safety of LABA has been challenged in recent years, especially when used without concomitant ICS (5). Although there is no conclusive evidence that addition of LABA could increase the risk of asthma-related hospitalizations or asthma mortality, the concern about its performance of enhancing airway remodeling still exists (6-9). Another meta-analysis also reported an increase in the risk of serious adverse events associated with LABA (10).
Both LTRAs and theophylline have anti-inflammatory effects (11,12). There is some evidence to support the synergistic effect of these two add-on therapies at the cellular or pathophysiology level. LTRAs inhibit the production of cysteinyl leukotrienes, important pro-inflammatory mediators in asthma that are unaffected by steroid treatment (12-14). Theophylline may reduce mucosal permeability and attenuate development of asthma inflammation after allergen challenge (15). Many clinical trials have also shown the addition of LTRA or theophylline to be effective in asthma treatment (16-18). Thus, both addition of LTRAs or theophylline may potentiate the anti-inflammatory effect of ICS and lead to better asthma control. A number of trials aimed to compare these two add-on therapies have been carried out over the last decade, but these studies had very small sample of patients in both groups. Therefore, we evaluated the relative benefits and safety profile of adding either LTRAs or theophylline to ICS in patients with symptomatic asthma in a systematic manner.
Methods
Data sources
We searched PubMed, Excerpta Medica Database (EMBASE), ScienceDirect, ClinicalTrials.gov, Chinese Biomedical Database and the Cochrane Central Register of Controlled Trials (CENTRAL) for potentially relevant articles published until Nov 2014, with no lower date limit applied. The following search strategy was used: “leukotriene receptor antagonist OR montelukast OR pranlukast OR zafirlukast” and “theophylline” and “steroid OR ICSs OR budesonide OR beclomethasone OR fluticasone” and “asthma”. These searches were supplemented by hand searching of leading respiratory journals and conference abstracts. Reference lists were searched for additional articles.
Study selection
Two reviewers (X Chen and YB Kang) screened the title, abstract or citations and excluded all studies that clearly did not fit the inclusion criteria. Studies included in the meta-analysis met the following criteria: (I) studies should be RCTs conducted in asthmatic adults or children in whom LTRAs or theophylline were added, as a fixed dose combination, to ICS; (II) despite being treated with ICS, patients had asthmatic symptoms prior to study entry or during the run-in period; (III) the intervention must have been administered for a minimum of 4 weeks. Both blinded and non-blinded trials were included.
Data extraction and quality assessment
Data for the trials were extracted by two reviewers independently. If disagreement arose, all the authors conferred till a consensus was arrived at. The extracted data included the characteristics, study design and outcomes from papers. The primary outcomes were changes in lung function from baseline, including forced expiratory volume in the first second (FEV1) and peak expiratory flow (PEF). The secondary outcomes were the number of adverse events reported, the use of rescue medication and asthma exacerbations.
The RCTs included in our meta-analysis were assessed for methodological quality by using the 5-point scale (0= worst and 5= best) described by Jadad et al. (19). The maximum score that could be awarded to a trial was five points and a score higher than 2 was considered to be indicative of adequate methodological quality (20).
Statistical analysis
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (21). All included trials were combined using Review Manager 5.2 (The Cochrane Collaboration, Software Update, Oxford, UK). For dichotomous variables, we combined data as risk ratio (RR) with 95% confidence interval (CI). For continuous outcomes, such as pulmonary function tests, we combined data as mean difference (MD) with 95% CI. Chi-square-based Q-statistic test and I2 test were applied to assess the homogeneity of effect sizes between studies. I2>50% or P<0.10 represent the cut-off level for statistical significance respectively. In the absence of heterogeneity, we used a fixed-effect model. If heterogeneity was suggested, a random-effects model was chosen. Sensitivity analysis was conducted on a statistical method of analysis (random vs. fixed effects model).
Results
Study selection and methodological quality
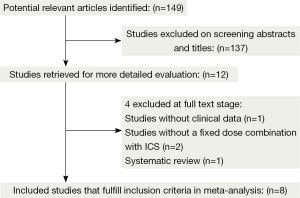
The flowchart shows the detailed selection process according to PRISMA guideline (Figure 1). Initially, 149 articles were identified from the literature searches and we excluded 137 that were either not relevant or had duplicate data. Twelve full-text articles were reviewed for detail evaluation. Of these, four trials were further excluded according to the inclusion criteria (22-25). Finally, eight RCTs fulfilled the inclusion criteria and were selected for meta-analysis (26-33). Using the methods of Jadad et al., five studies were found to have a Jadad score of 3-5 and three studies were found to have a Jadad score of 2. We established a database according to the extracted information from each study.
Study characteristics
The inclusion criteria and study characteristics are given in Tables 1 and 2. Six studies focused on adults and two on children exclusively. A total of 160 children and 300 adults were recruited in the studies. The patients in all of the studies showed symptoms before randomized to treatment.
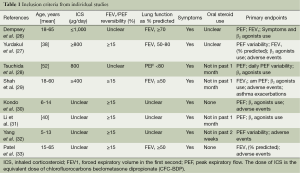
Full table
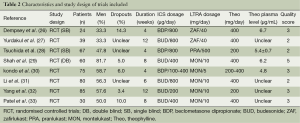
Full table
Primary outcomes
Changes from baseline in morning PEF
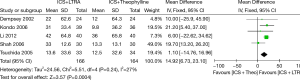
Six trials examined mean morning PEF and five studies contributed data to this analysis (330 participants including 75 children and 255 adults). Addition of LTRA resulted in a significantly greater improvement in morning PEF than addition of theophylline [MD 16.94 (95% CI: 11.49-22.39) L/min, P<0.00001, I2=28%] (Figure 2).
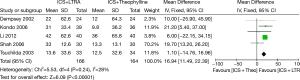
Changes from baseline in evening PEF
Four studies were considered for this analysis (270 participants including 75 children and 195 adults). There was no significant difference between these two therapies in improving evening PEF in asthmatics [MD 6.01 (95% CI: −2.85 to 14.87) L/min, P=0.18, I2=24%] (Figure 3).
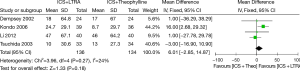
Changes from baseline in FEV1 (L)
Two studies contributed data to changes in FEV1 (including 108 adults). ICS plus LTRA was superior to ICS plus theophylline therapy in improving FEV1 in asthmatics [MD 0.09 (95% CI: 0.03-0.15) L, P=0.005, I2=0%] (Figure 4).
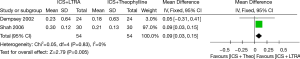
Sensitivity analysis
To evaluate the sensitivity of meta-analysis, we calculated the random effect model for morning PEF. The random effect model of morning PEF showed a pooled MD of 14.92 (95% CI: 6.73-23.10), similar to the result [MD 16.94 (95% CI: 11.49-22.39)] obtained from the fixed-effect model (Figure 5).
Secondary outcomes
Adverse events
Five studies with 297 participants (including 168 children and 129 adults) reported adverse events, and their results showed that there were no statistical differences in adverse events between the groups [RR 1.31 (95% CI: 0.77-2.24), P=0.32, I2=0%] (Figure 6).
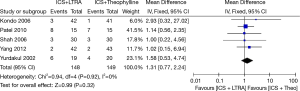
Decrease in rescue medication use (puffs/day)
Four studies were considered for this analysis (212 participants including 46 children and 166 adults). There was no significant difference between these two therapies in the use of rescue medication [MD −0.01 (95% CI: −0.21 to 0.19), P=0.70, I2=0%].
Asthma exacerbations
Two studies in 143 participants (including 83 children and 60 adults) contributed data for this outcome. There was no significant difference between the two treatments [RR 1.00 (95% CI: 0.31-3.20), P=0.99, I2=0%].
Discussion
Currently, LTRAs are generally considered to be of more importance than theophylline in the therapies for asthma. LTRA monotherapy is recommended as the alternative option at step 2 according to Global Initiative for Asthma (GINA) while theophylline has become a third-line treatment in many industrialized countries (2). Even so, theophylline is still widely used in China, especially in its less developed areas. The Chinese clinicians prefer to adopt the regimen lower than routine dose and many of them value its additional effects (34,35). A meta-analysis combined four RCTs including 182 asthmatic patients reported that ICS plus LTRA results in greater improvement in pulmonary function test parameters than ICS plus theophylline (25). However, more trials comparing these two therapies have been conducted since the previous review was carried out, which include studies conducted in children and Chinese people. In the light of these new evidences, it is necessary to reassess the efficacy and safety of ICS-LTRA as compared to ICS-theophylline.
The results of our meta-analysis indicate that addition of LTRA results in more improvement in both morning PEF (MD 16.94 L/min, 95% CI: 11.49-22.39) and FEV1 (MD 0.09 L, 95% CI: 0.03-0.15) as compared to addition of theophylline in patients who remain symptomatic with the prescription of low to moderate doses of ICS. There was no statistical heterogeneity, and the sensitivity analysis showed that the random and fixed effect models for morning PEF had similar results. No difference in evening PEF was found between these two therapies. This may relate to the fact that some of the studies did not present data suitable for meta-analysis. However, data on other clinically relevant outcomes that could be pooled were sparse. In fact, the included individual studies failed to prove any superiority of LTRA than theophylline with regard to asthma related symptoms, quality of life and exacerbations. Further investigations aimed at the effects of ICS-LTRA versus ICS-theophylline on clinically relevant outcomes are required.
No significant statistical difference was found in adverse events between the two therapies. A major limitation of theophylline is the high frequency of adverse effects, such as headache, nausea, abdominal discomfort and cardiac arrhythmias (36). However, few adverse events were observed in the theophylline groups of included studies. This could be interpreted as a result of the low doses of theophylline that give plasma concentrations of 5 to 10 µg/mL, coinciding with the anti-inflammatory target range but having less adverse reaction. The results of meta-analysis showed that there were no statistical differences in need for rescue medication between the two treatments during the study period. This was also supported by the study conducted by Yurdakul et al. (27), which had a relatively long follow-up period by 3 months among the included studies. Two of the included trials reported asthma exacerbation rates and the results showed that there were no statistical differences between the groups. The conclusion of our meta-analysis is similar to the study conducted by Fang et al. (25) and we found that it may be applied to children.
Leucotriene receptor antagonists are a relatively new class of antiasthmatic drugs while theophylline is an old drug that has been used for over 70 years. Both LTRAs and theophylline have the advantage of being administered orally. Many studies indicated that adding LTRAs to ICS significantly improved lung function and asthmatic symptoms in comparison with increasing the dose of ICS (37-39). LTRAs could also increase patient compliance and bring remarkable ease of anti-inflammatory treatment administration (40). Furthermore, LTRAs are well tolerated, and few if any class-related effects have so far been recognized (2). The results of our study indicate that LTRAs show greater beneficial effects in increasing PEF and FEV1 compared with theophylline. LTRAs could be a good choice as add-on therapy for asthmatic patients treated with low to moderate ICS. However, oral sustained release theophylline may also be an attractive steroid-sparing agent considering its lower costs and efficacy, especially in developing countries (41). The adverse effects of theophylline generally occur over the serum concentration range of 15 to 20 ug/mL and can be significantly decreased by given at low doses. It’s worth mentioning that in such patients the withdrawal of sustained release theophylline has been associated with deterioration of control (42).
There were several limitations in this meta-analysis. First, some of the studies did not present data suitable for meta-analysis so that we could not investigate whether or not agents (different LTRAs or different ICSs), or duration of treatment affect the evaluation of these two therapies. For the same reason, the subgroup analyses on children versus adults could not be examined. So the results of this study are more suitable for adults. Second, the funnel plot was not created for evaluation of the publication bias owning to the limited studies available for meta-analysis. So there exists a possibility of publication bias in this meta-analysis. Despite these limitations, we believe that these pooled results provide helpful information.
Conclusions
Our meta-analysis suggests that the combination of LTRA and ICS leads to modestly greater improvement in lung function than the combination of theophylline and ICS. But no statistically significant differences are found in rescue medication use, adverse effects and asthma exacerbation between the two therapies. More randomized controlled trials in the form of large sample and long duration are required due to our study limitations.
Acknowledgements
Funding: This study was funded by the Guangdong Provincial Science and Technology Project (2011B080701062), (2013B022000072) and Guangdong Natural Science Foundation (S2012010008623).
Authors’ contributions: Conceived and designed the experiments: R Chen, LQ Wang. Selected studies and extracted data: X Chen, YB Kang, Y Li. Analyzed the data: X Chen, YB Kang, Y Li, YW Luo, Z Zhu. Wrote the paper: YB Kang, X Chen, R Chen.
Disclosure: The authors declare no conflict of interest.
References
- Fanta CH. Asthma. N Engl J Med 2009;360:1002-14. [PubMed]
- Global Initiative for Asthma (GINA): global strategy for asthma management and prevention (revised 2014). Available online: http://www.ginasthma.org
- Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database Syst Rev 2010;CD005535. [PubMed]
- Agarwal R, Khan A, Aggarwal AN, et al. Is the SMART approach better than other treatment approaches for prevention of asthma exacerbations? A meta-analysis. Monaldi Arch Chest Dis 2009;71:161-9. [PubMed]
- Cates CJ, Jaeschke R, Schmidt S, et al. Regular treatment with formoterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database Syst Rev 2013;6:CD006924. [PubMed]
- Vanacker NJ, Palmans E, Pauwels RA, et al. Effect of combining salmeterol and fluticasone on the progression of airway remodeling. Am J Respir Crit Care Med 2002;166:1128-34. [PubMed]
- Weatherall M, Wijesinghe M, Perrin K, et al. Meta-analysis of the risk of mortality with salmeterol and the effect of concomitant inhaled corticosteroid therapy. Thorax 2010;65:39-43. [PubMed]
- Nelson H, Bonuccelli C, Radner F, et al. Safety of formoterol in patients with asthma: combined analysis of data from double-blind, randomized controlled trials. J Allergy Clin Immunol 2010;125:390-396.e8.
- Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006;129:15-26. [PubMed]
- Salpeter SR, Buckley NS, Ormiston TM, et al. Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths. Ann Intern Med 2006;144:904-12. [PubMed]
- Weinberger M, Hendeles L. Theophylline in asthma. N Engl J Med 1996;334:1380-8. [PubMed]
- Drazen JM, Israel E, O'Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med 1999;340:197-206. [PubMed]
- Krawiec ME, Jarjour NJ. Leukotriene receptor antagonists. Semin Respir Crit Care Med 2002;23:399-410. [PubMed]
- Pizzichini E, Leff JA, Reiss TF, et al. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. Eur Respir J 1999;14:12-8. [PubMed]
- Tee AK, Koh MS, Gibson PG, et al. Long-acting beta2-agonists versus theophylline for maintenance treatment of asthma. Cochrane Database Syst Rev 2007;CD001281. [PubMed]
- Evans DJ, Taylor DA, Zetterstrom O, et al. A comparison of low-dose inhaled budesonide plus theophylline and high-dose inhaled budesonide for moderate asthma. N Engl J Med 1997;337:1412-8. [PubMed]
- Ukena D, Harnest U, Sakalauskas R, et al. Comparison of addition of theophylline to inhaled steroid with doubling of the dose of inhaled steroid in asthma. Eur Respir J 1997;10:2754-60. [PubMed]
- Laviolette M, Malmstrom K, Lu S, et al. Montelukast added to inhaled beclomethasone in treatment of asthma. Montelukast/Beclomethasone Additivity Group. Am J Respir Crit Care Med 1999;160:1862-8. [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [PubMed]
- Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609-13. [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [PubMed]
- Cai YH, Lu HT, Feng KR, et al. Comparison the effect of treatment of inhaled corticosteroids combined with small-dose theophylline or leukotriene modifiers on asthma children under age 5. China Modern Medicine 2014;21:103-6.
- Yurdakul AS, Taci N, Eren A, et al. Comparative efficacy of once-daily therapy with inhaled corticosteroid, leukotriene antagonist or sustained-release theophylline in patients with mild persistent asthma. Respir Med 2003;97:1313-9. [PubMed]
- American Lung Association Asthma Clinical Research Centers. Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. Am J Respir Crit Care Med 2007;175:235-42. [PubMed]
- Fang H, Wang J, Jin D, et al. Comparison of leukotriene receptor antagonist and theophylline in addition to inhaled corticosteroid in adult asthma: a meta-analysis. Biomol Ther 2011;19:296-301.
- Dempsey OJ, Fowler SJ, Wilson A, et al. Effects of adding either a leukotriene receptor antagonist or low-dose theophylline to a low or medium dose of inhaled corticosteroid in patients with persistent asthma. Chest 2002;122:151-9. [PubMed]
- Yurdakul AS, Calişir HC, Tunçtan B, et al. Comparison of second controller medications in addition to inhaled corticosteroid in patients with moderate asthma. Respir Med 2002;96:322-9. [PubMed]
- Tsuchida T, Matsuse H, Machida I, et al. Evaluation of theophylline or pranlukast, a cysteinyl leukotriene receptor 1 antagonist, as add-on therapy in uncontrolled asthmatic patients with a medium dose of inhaled corticosteroids. Allergy Asthma Proc 2005;26:287-91. [PubMed]
- Shah AR, Sharples LD, Solanki RN, et al. Double-blind, randomised, controlled trial assessing controller medications in asthma. Respiration 2006;73:449-56. [PubMed]
- Kondo N, Katsunuma T, Odajima Y, et al. A randomized open-label comparative study of montelukast versus theophylline added to inhaled corticosteroid in asthmatic children. Allergol Int 2006;55:287-93. [PubMed]
- Li Y, Ding J. Comparative study of inhaled corticosteroid combined with theophylline or with montelukast in treatment of asthma. China Practical Journal of Medicine 2012;39:23-4.
- Yang ZN, Zhu XM, Yu XY. Slow-release theophylline or montelukast respectively combined with inhaled budesonide in the treatment of children with cough variant asthma: Comparative study. Practical Journal of Medicine & Pharmacy 2012;29:197-9.
- Patel YA, Patel P, Bavadia H, et al. A randomized, open labeled, comparative study to assess the efficacy and safety of controller medications as add on to inhaled corticosteroid and long-acting β2 agonist in the treatment of moderate-to-severe persistent asthma. J Postgrad Med 2010;56:270-4. [PubMed]
- Wang Y, Wang CZ, Lin KX, et al. Comparison of inhaled corticosteroid combined with theophylline and double-dose inhaled corticosteroid in moderate to severe asthma. Respirology 2005;10:189-95. [PubMed]
- Li J, Mo H, Huang H. Effect of low dose of inhaled corticosteroid combined with small dose of oral theophylline on treatment of bronchial asthma. Zhonghua Jie He He Hu Xi Za Zhi 2000;23:336-9. [PubMed]
- Barnes PJ. Theophylline: new perspectives for an old drug. Am J Respir Crit Care Med 2003;167:813-8. [PubMed]
- Joos S, Miksch A, Szecsenyi J, et al. Montelukast as add-on therapy to inhaled corticosteroids in the treatment of mild to moderate asthma: a systematic review. Thorax 2008;63:453-62. [PubMed]
- Price DB, Hernandez D, Magyar P, et al. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax 2003;58:211-6. [PubMed]
- Virchow JC Jr, Prasse A, Naya I, et al. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am J Respir Crit Care Med 2000;162:578-85. [PubMed]
- Amlani S, Nadarajah T, McIvor RA. Montelukast for the treatment of asthma in the adult population. Expert Opin Pharmacother 2011;12:2119-28. [PubMed]
- Wang Y, Lin K, Wang C, et al. Addition of theophylline or increasing the dose of inhaled corticosteroid in symptomatic asthma: a meta-analysis of randomized controlled trials. Yonsei Med J 2011;52:268-75. [PubMed]
- Baba K, Sakakibara A, Yagi T, et al. Effects of theophylline withdrawal in well-controlled asthmatics treated with inhaled corticosteroid. J Asthma 2001;38:615-24. [PubMed]