A novel association of adenosine deaminase with paroxysmal atrial fibrillation: a propensity score analysis from a case-control study
Introduction
With the advent of catheter ablation (1) and new oral anticoagulants for atrial fibrillation (2-4), the treatment of atrial fibrillation has made much progress recently. However, atrial fibrillation is still a common cardiac rhythm disturbance in clinical practice and an important indicator of morbidity and mortality, and increases in prevalence with advancing age. Atrial fibrillation is usually classified into three forms of paroxysmal, persistent and permanent (5). In several studies, patients who develop sustained forms of atrial fibrillation (persistent/permanent) have higher rates of cardiovascular disease (CVD) morbidity and mortality than those who develop paroxysmal atrial fibrillation (PAF). PAF is a common form at the early onset stage, particularly within the 1st year of diagnosis, which will progress to sustained forms of atrial fibrillation if not timely or properly approached (6). Therefore, identification of factors that predispose to PAF may play an important role in lowering atrial fibrillation-related morbidity and improving response to traditional therapies.
Prior work has indentified age (6-8), body mass index (9,10), underlying heart disease (11,12), and other comorbidities such as chronic obstructive pulmonary disease and hypertension as risk factors for atrial fibrillation (8,11). To date, studies have examined single baseline measures of traditional risk factors, and data on biomarker associations are lacking. We sought to explore novel biochemical measures possibly associated with PAF after balancing the traditional risk factors.
Methods
Ethics statement
All patient records were anonymized and de-identified prior to analysis, and the Institutional Review Board waived the need for informed consent due to the retrospective nature of this study. This study protocol has been approved by the Institutional Review Board of Soochow University and conforms to the principals outlined in the Declaration of Helsinki.
Patients
Consecutive patients aged ≥18 years that were hospitalized in the department of cardiology, the First Affiliated Hospital of Soochow University from 1st Jan. 2010 through 31st Dec. 2013 for PAF and for health checkup were included. Clinical and lab data for initial medical contacts were collected for those with multiple hospitalizations or checkups. Patients with PAF usually had more than two diagnoses and were in bad condition as compared with those hospitalized for health check-ups. Therefore, patients’ selection in PAF group was limited to those with at most two diagnoses so as to increase the success rate of matching. Exclusion criteria included thyroid dysfunction, severe liver function abnormalities, chronic renal failure, acute coronary syndrome within 1 month, acute stroke within 1 month, congenital heart diseases, rheumatic or prosthetic valvular heart diseases, pulmonary stenosis, acute or chronic respiratory failure and coexistence of atrial fibrillation and paroxysmal supraventricular tachycardia.
Definition of PAF
A patient has had two or more episodes, and atrial fibrillation is considered as recurrent. If recurrent atrial fibrillation terminates by itself, it is designated paroxysmal. Termination by pharmacological therapy or electrical cardioversion before expected spontaneous termination less than 7 days does not change the designation paroxysmal (5). The atrial fibrillation diagnosis was based on electrocardiogram during the hospitalization period.
Diagnoses and definitions
Primary hypertension is diagnosed based on the systolic and/or diastolic blood pressure ≥140/90 mmHg or on confirmatory past history now on antihypertensive treatments (13); type 2 diabetes mellitus is diagnosed based on a plasma glucose ≥7.0 mmol/L after fasting for at least 8 hours or on 2-hour plasma glucose ≥11.1 mmol/L during OGTT or on a random plasma glucose ≥11.1 mmol/L in patients with classic symptoms of hyperglycemia or on hyperglycemic crisis or on a confirmatory past history now on anti-hyperglycemic treatments (14); dyslipidemias are diagnosed based on the total cholesterol level ≥6.22 mmol/L or on the low density cholesterol level ≥4.14 mmol/L or on the high density cholesterol level ≥1.55 mmol/L or ≤1.04 mmol/L, or on the triglyceride ≥2.26 mmol/L (15); coronary artery diseases are diagnosed based on the confirmatory myocardial infarction history or on diameter stenosis of one of major coronary arteries ≥50% on angiography; pulmonary diseases include acute pulmonary infection, pulmonary malignancies, chronic obstructive airway diseases and pneumothorax; other CVDs include sick sinus disease, sinus bradycardia, mitral stenosis, hypertrophic myocardiopathy, congenital heart diseases, paroxysmal atrial tachycardia, ventricular tachycardia and coronary artery atherosclerosis; the miscellaneous include acute tonsillitis, chronic gastritis, syphilis, depression, urinary tract infection, acute upper respiratory tract infection and rheumatoid arthritis. The presence of the above mentioned diseases is considered as positive and calculated as percentages.
Transthoracic echocardiography
Transthoracic echocardiography was performed by experienced echocardiologists on all patients for obtaining echo parameters, such as ejection fraction, tricuspid pressure gradient in systole, root aortic diameter, right ventricular diameter, right atrial diameter, left atrial diameter, ventricular septal thickness, left ventricular posterior wall thickness, left ventricular end-systolic diameter and left ventricular end-diastolic diameter; for obtaining ranked parameters, such as aortic regurgitation, tricuspid regurgitation and mitral regurgitation. Slight, mild, moderate and severe regurgitation judged by echocardiologists was recorded as 0.5, 1, 2 or 3, respectively and no regurgitation judged by echocardiologists was recorded as 0. Mild to moderate regurgitation judged by echocardiologists was recorded as (1+2)/2=1.5. Moderate to severe regurgitation would be addressed in the same manner.
Biochemical marker examination
Venous blood sample taking was done in the morning after 8-hour fasting to examine the serum lipid profile, biochemical markers and blood glucose levels using those routine methods according to the products specifications.
Statistical analysis
Categorical or ordered variables are presented as frequencies or percentages, and unadjusted comparisons were performed using χ2 or Fisher exact or Cochrane-Mantel-Haenszel (CMH) tests where appropriate. Continuous variables are presented as mean ± SD or median [IQR (interquartile range)], and unadjusted comparisons were made using independent-sample t-tests or Wilcoxon rank-sum test.
We used the propensity score method to mitigate the influence of the nonrandom selection of PAF and non-PAF patients. The propensity score for an individual is defined as the conditional probability of the presence of PAF given the individual’s covariates. To estimate these scores, we created a logistic regression model on the following covariates: (I) demographic variables, such as age, sex, body weight, height, systolic and diastolic blood pressure at admission; (II) clinical variables, such as primary hypertension, type 2 diabetes mellitus, dyslipidemia, coronary artery disease, other CVDs, pulmonary diseases, and the miscellaneous.
We performed a one-to-one nearest neighbor match on the logit of the propensity score without a caliper. Percent bias calculations and t-tests were applied for balancing check of covariates both before and after matching. For good balancing, t-tests for equality of means should be no significant after matching, and the standardized bias should be less than 5% after matching. Paired t-test or sign-rank test or χ2 test or Fisher exact test were used where appropriate for propensity-score matched data. Univariate logistic regression and multivariate stepwise logistic regression were performed on those significant variables found by paired t-test or sign-rank test. Significance level for removal from and addition to the model were preset at 0.1 and 0.05, respectively. Odds ratios (ORs) and 95% confidence intervals (CIs) were reported. Statistical significance was defined as P<0.05. All statistical analyses were performed using Stata 12.0.
Results
A total of 1,802 eligible patients were identified between 1st Jan. 2010 and 31st Dec. 2013. A total of 895 patients had at least 1 exclusion criterion. After excluding these patients, the total analytic cohort numbered 907 patients. Of these, 779 patients were for health checkup, and 128 patients were diagnosed as PAF. Propensity score matching was used to obtain a balanced cohort of 124 patients per group.
After-matching balancing check for covariates based on which propensity scores were estimated
The t-test is not significant for all means of covariates after matching. The absolute values of %bias for most covariates were around 5% with the only two exceptions of sex and diastolic pressure, showing a small unbalance of 9.8% and 9.7%, respectively. The overall mean %bias is 4% after matching. See Table 1.
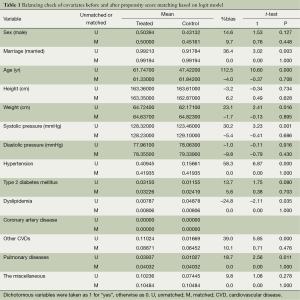
Full table
Baseline demographic and clinical variables before and after propensity score matching
Patient characteristics for the unadjusted and propensity score-matched patients are given in Table 2. As many as eight covariates were significantly different (P<0.05) between the both groups before matching. However, all covariates were comparable and well balanced (P>0.05) between the both groups after propensity score matching.
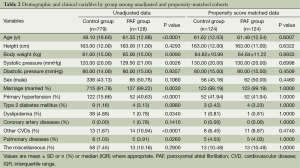
Full table
Echocardiography parameters before and after propensity score matching
Before propensity score matching of demographic and clinical variables, most echocardiography parameters were significantly different (P<0.05) between the PAF group and the non-PAF group except those of ejection fraction, septal thickness and left ventricle (LV) end-systolic, end-diastolic diameter. After propensity score matching, most echocardiography parameters were well-balanced and showed no significant differences (P>0.05) between the both groups while only five parameters of tricuspid pressure gradient, right atrial diameter, left atrial diameter, mitral valvular regurgitation and tricuspid regurgitation were significantly different (P<0.05). See Table 3.
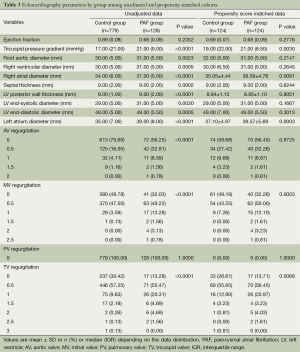
Full table
Values are mean ± SD or n (%) or median (IQR) depending on the data distribution. PAF, paroxysmal atrial fibrillation; LV, left ventricle; AV, aortic valve; MV, mitral valve; PV, pulmonary valve; TV, tricuspid valve; IQR, interquartile range.
Lipid profile and biochemical markers before and after propensity score matching
Before propensity score matching of demographic and clinical variables, up to 13 biochemical markers of γ-glutamyl transferase, blood urea nitrogen, uric acid, total bilirubin, total protein, albumin, direct bilirubin, phosphorus, creatinine, high sensitive C-reactive protein, calcium, sodium and indirect bilirubin were significantly different (P<0.05) between the PAF group and the non-PAF group. After propensity score matching, most biochemical markers were well balanced and only four markers of prealbumin, aspartic aminotransferase, creatinine and adenosine deaminase (ADA) were significantly different (P=0.0440, P=0.0371, P=0.0149 and P=0.0091, respectively) between the both groups. See Table 4.
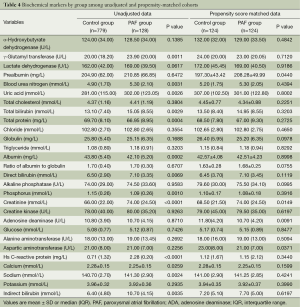
Full table
ORs for PAF using logistic regression analysis
Univariate logistic regression analysis was performed on tricuspid pressure gradient, right atrial diameter, left atrial diameter, creatinine, ADA, prealbumin, aspartic aminotransferase, mitral valvular regurgitation and tricuspid regurgitation that were significantly different by univariate analysis between the both groups. Risk factors with significant differences for PAF included tricuspid pressure gradient (OR =1.0409, P=0.002, 95% CI: 1.0152-1.0674), right atrial diameter (OR =1.0763, P=0.010, 95% CI: 1.0176-1.1384), left atrial diameter (OR =1.09, P=0.001, 95% CI: 1.04-1.15), mitral valvular regurgitation (OR =3.4611, P=0.001, 95% CI: 1.7000-7.0467), tricuspid regurgitation (OR =1.0409, P=0.002, 95% CI: 1.0152-1.0674) and ADA (OR =0.9160, P=0.015, 95% CI: 0.8536-0.9829). See Table 5.

Full table
Risk factors, which remained to be kept in multivariate stepwise logistic regression model, included ADA (OR =0.9160, P=0.015, 95% CI: 0.8536-0.9829), mitral valvular regurgitation (OR =3.4611, P=0.001, 95% CI: 1.7000-7.0467) and left atrial diameter (OR =1.0913, P=0.001, 95% CI: 1.0387-1.1465). See Table 5.
Discussion
We have demonstrated that the ADA was associated with PAF. To the best of our knowledge, this is the first study to report a novel association of ADA with PAF. Every 1 U/L increase of ADA corresponded to reduction of the PAF by about 8% in the current study. The well-established demographic and clinical risk factors for atrial fibrillation included age (6-8), body mass index (9,10), underlying heart disease (16), hypertension and chronic obstructive pulmonary disease (8,11); the biochemical marker risk factors for atrial fibrillation included hemoglobin A1c (17), glomerular filtration rate (18), C-reactive protein (19), and serum albumin levels (20), etc. We used the propensity score method to mitigate the impacts of traditional demographic and clinical covariates in order to ensure the reliability of biochemical marker results in the current study. The demographic and clinical covariates were well balanced, and a relative part of biochemical markers and echocardiography parameters was also balanced after propensity matching. As well as the ADA, the traditional risk factors, such as tricuspid pressure gradient, right atrial diameter, left atrial diameter, mitral valvular regurgitation, tricuspid regurgitation and creatinine were also identified as risk factors for atrial fibrillation in the current study, which was consistent with previous studies (18,21,22). That the traditional risk factors have been retained in the current study has in turn confirmed the reliability of the current study.
Stepwise multivariate logistic regression analysis revealed that ADA, mitral valvular regurgitation and left atrial diameter remained to be independent risk factors for the occurrence of PAF, among which the adenosine was the only protective factor for the occurrence of PAF.
The ADA is an important enzyme with three family members of ADA 1, 2 and L (23), of which, the ADA 2 is the most abundant in human plasma (24). The ADA levels reflect cellular immune functionality (25) and are also closely associated with CVDs (26-28). Adenosine, a degradation product of ATP, has been attributed to exert different effects on heart: a protective agent for reperfusion heart on one hand (29-31) and a harmful agent for induction of some heart diseases such as atrial fibrillation and atrial flutter on the other hand (32-34). The induction of atrial fibrillation by adenosine has been well recognized and the possible underlying mechanisms include sympathoexcitatory effects, direct stimulatory effects on pulmonary vein tissue and the shortening of atrial action potential duration (32). This at least means that the adenosine has a property of double-edged sword and that the maintenance of the adenosine at an appropriate level is important. The ADA can regulate intra- and extracellular levels of adenosine through hydrolytic deaminase to inosine. That the lower adenosine concentrations resulting from the relatively higher ADA prolong the atrial action potential duration and decrease the sympathetic nerve activity may explain, at least in part, the higher ADA concentration as a protective factor for PAF revealed in the current study.
Limitations
A case-control study design is potentially subjected to confounding factors if there is differential ascertainment of risk factors between cases and controls. We minimized this factor by using standardized methods for data collection in both cases and controls. A selection bias of controls is also an issue in a case-control study that has to be addressed. We minimized these factors using propensity score matching. Some of the risk factors were ascertained or measured based on history or self-report and therefore ascertained with some error. The actual blood pressure value is potentially confounded because it might have fallen in some patients as a result of the drug used. Similarly, glucose concentrations rise and fall as a result of diet and drug used and are therefore not indication of earlier levels. In our study, the patients’ past histories, blood pressure values and glucose concentrations were all recorded as clinical variables for statistical analysis so as to minimize this possible cofounding factor.
In conclusion, our study has shown that the ADA, as a protective factor, seems to be associated with PAF. The current study provides new insights into the prevention and treatment of PAF. A prospective, randomized controlled study should be designed for further confirming this association. The remaining six traditional risk factors are also identified in the current study, which suggest that modification of traditional risk factors should not be ignored.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528-606. [PubMed]
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. [PubMed]
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91. [PubMed]
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92. [PubMed]
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:e199-267. [PubMed]
- Kerr CR, Humphries KH, Talajic M, et al. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J 2005;149:489-96. [PubMed]
- Jahangir A, Lee V, Friedman PA, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation 2007;115:3050-6. [PubMed]
- de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725-31. [PubMed]
- Tsang TS, Barnes ME, Miyasaka Y, et al. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J 2008;29:2227-33. [PubMed]
- Dublin S, French B, Glazer NL, et al. Risk of new-onset atrial fibrillation in relation to body mass index. Arch Intern Med 2006;166:2322-8. [PubMed]
- Thomas MC, Dublin S, Kaplan RC, et al. Blood pressure control and risk of incident atrial fibrillation. Am J Hypertens 2008;21:1111-6. [PubMed]
- Yu K, Xing A, Wang D, et al. Prevalence and relative risk factors of atrial fibrillation in male coal miners in North China. Int J Cardiol 2014;174:223-4. [PubMed]
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507-20. [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33 Suppl 1:S62-9. [PubMed]
- Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi 2007;35:390-419. [PubMed]
- Kato T, Yamashita T, Sagara K, et al. Progressive nature of paroxysmal atrial fibrillation. Observations from a 14-year follow-up study. Circ J 2004;68:568-72. [PubMed]
- Sandhu RK, Conen D, Tedrow UB, et al. Predisposing factors associated with development of persistent compared with paroxysmal atrial fibrillation. J Am Heart Assoc 2014;3:e000916. [PubMed]
- Alonso A, Lopez FL, Matsushita K, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011;123:2946-53. [PubMed]
- Cao JJ, Thach C, Manolio TA, et al. C-reactive protein, carotid intima-media thickness, and incidence of ischemic stroke in the elderly: the Cardiovascular Health Study. Circulation 2003;108:166-70. [PubMed]
- He YM, Yang XJ, Hui J, et al. Low serum albumin levels in patients with paroxysmal atrial fibrillation: what does it mean? Acta Cardiol 2006;61:333-7. [PubMed]
- Acar G, Akçay A, Doğan E, et al. The prevalence and predictors of atrial fibrillation in hemodialysis patients. Turk Kardiyol Dern Ars 2010;38:8-13. [PubMed]
- Zhou X, Otsuji Y, Yoshifuku S, et al. Impact of atrial fibrillation on tricuspid and mitral annular dilatation and valvular regurgitation. Circ J 2002;66:913-6. [PubMed]
- Rosemberg DB, Rico EP, Guidoti MR, et al. Adenosine deaminase-related genes: molecular identification, tissue expression pattern and truncated alternative splice isoform in adult zebrafish (Danio rerio). Life Sci 2007;81:1526-34. [PubMed]
- Iwaki-Egawa S, Yamamoto T, Watanabe Y. Human plasma adenosine deaminase 2 is secreted by activated monocytes. Biol Chem 2006;387:319-21. [PubMed]
- Zavialov AV, Gracia E, Glaichenhaus N, et al. Human adenosine deaminase 2 induces differentiation of monocytes into macrophages and stimulates proliferation of T helper cells and macrophages. J Leukoc Biol 2010;88:279-90. [PubMed]
- Gloria-Bottini F, Banci M, Saccucci P, et al. The interaction of ACP1, ADA1, diabetes and gender in coronary artery disease. Am J Med Sci 2010;340:103-8. [PubMed]
- Navon Elkan P, Pierce SB, Segel R, et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med 2014;370:921-31. [PubMed]
- Saccucci P, Binczak-Kuleta A, Banci M, et al. Coronary artery disease. A study of three polymorphic sites of adenosine deaminase gene. Acta Cardiol 2014;69:39-44. [PubMed]
- Donato M, Gelpi RJ. Adenosine and cardioprotection during reperfusion--an overview. Mol Cell Biochem. 2003;251:153-9. [PubMed]
- Kaul A, Misra MK, Sethi R. Evaluation of the roles of adenosine deaminase and xanthine oxidase in reperfusion injury in patients with myocardial infarction. Clin Chim Acta 2007;380:225-7. [PubMed]
- Keeling IM, Obermayr RP, Schneider B, et al. Postischemic cardiac function recovery in the isolated rat heart: effects of adenosine deaminase and nucleoside transport inhibition. Langenbecks Arch Surg 2000;385:531-7. [PubMed]
- Ip JE, Cheung JW, Chung JH, et al. Adenosine-induced atrial fibrillation: insights into mechanism. Circ Arrhythm Electrophysiol 2013;6:e34-7. [PubMed]
- Cheung JW, Chung JH, Ip JE, et al. Time course of adenosine-induced pulmonary vein reconnection after isolation: implications for mechanism of dormant conduction. Pacing Clin Electrophysiol 2012;35:556-63. [PubMed]
- Kabell G, Karas BJ, Corbisiero R, et al. Effects of adenosine on wavelength of premature atrial complexes in patients without structural heart disease. Am J Cardiol 1996;78:1443-6. [PubMed]


