Effects of bronchoscopic lung volume reduction using transbronchial infusion of autologous blood and thrombin in patients with severe chronic obstructive pulmonary disease
Introduction
Existing medical treatments for the management of very severe chronic obstructive pulmonary disease (COPD) have limitations. It has been reported that lung volume reduction surgery (LVRS) improves the symptoms and prognosis of severe COPD when subjects are selected properly. However, increased short-term mortality after LVRS compared with that in the medical-therapy group was reported (1,2). Among patients whose forced expiratory volume in one second percent predicted (%FEV1) was less than 20%, the overall mortality rate was reported to be higher in patients who underwent LVRS than in patients who received medical treatment (1). It is very rare that LVRS is selected for therapy of COPD. However, the physical and emotional exhaustion of patients with very severe COPD compels the development of other palliative therapies.
In recent years, less invasive bronchoscopic approaches have been designed to achieve lung volume reduction in patients with severe emphysema. For example, the Zephyr® endobronchial valve (Pulmonx Inc, USA) showed safety and effectiveness in patients with heterogeneous emphysema in a randomized controlled multicenter trial (3).
Kobayashi and Kanoh reported the original method of bronchoscopic lung volume reduction (BLVR) involving transbronchial infusion of autologous blood and thrombin (4-6). We thought that their method is less invasive and applicable to very severe COPD patients. As their method is based on biological lung volume reduction (7,8) by scar formation after local inflammation, we thought that it is important to evoke enough, but not excessive, inflammation in the target emphysematous region.
The aims of this study were to evaluate the effectiveness and problems of BLVR using transbronchial infusion of autologous blood and thrombin (BLVR with blood) for the treatment of very severe COPD.
Materials and methods
Subjects
The subjects were three patients with very severe COPD whose dyspnea [Medical Research Council Dyspnea scale (MRC) grade 4] could not be relieved by maximum medical management, oxygen therapy and respiratory rehabilitation (2 days or more per week), and who strongly desired a treatment to relieve symptoms.
Methods
We targeted the most impaired lobe in the lung for BLVR with blood. The severity of the impairment was evaluated by chest CT, 99mTc-MAA lung perfusion scintigraphy and 133Xe lung ventilation scintigraphy. When the procedure was also applied to the contralateral lung, we set an interval of six months or longer between the procedures.
We obtained authorization to perform this study in advance by the Ethics Committee of the National Organization Himeji Medical Center (registration number 22-11, approval date: January 19, 2010). We obtained informed consent for treatment with the procedure and utilization of the data for research from all enrolled individuals.
BLVR with blood
BLVR with blood was performed under local anesthesia and mild sedation with 1.5-2 mg intravenous midazolam. A catheter (PW-1L-1; Olympus, Tokyo, Tapan) was inserted into the target bronchus in the emphysematous area through a flexible bronchoscope (BF-1T260; Olympus) under fluoroscopic guidance. Immediately after blood was drawn from the venous cannula that had been inserted into the patient’s median cubital vein, the blood (3-4 mL) was infused via the catheter followed by thrombin solution (2,500 units). The infusion was repeated in 4 to 6 regions mainly in the anterior segment of the target lobe (1 course).
We repeated the same procedure at intervals of several days until obvious infiltration (pneumonia) appeared in the emphysematous area.
Patients 1 and 2 underwent BLVR with blood in the right and left lungs at intervals of one year or six months, respectively, and patient 3 underwent the procedure in the right lung alone. We assessed the changes before and after BLVR with blood in pulmonary function, exercise capacity (3-minute walk), quality of life [St. George’s Respiratory Questionnaire (SGRQ)] (9), and range of motion of the diaphragm and doming of the diaphragm as assessed by the coronal view of CT images.
The 3-minute walk test was designed for the subjects in this study who found it difficult to keep walking for 6 minutes, and the test was stopped when the SpO2 fell to under 90% on continuous oxygen inhalation.
Results
The baseline data of the three subjects are presented in Table 1.
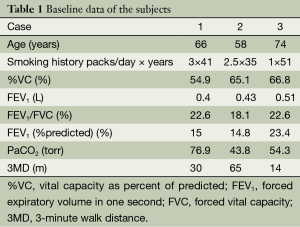
Full table
The duration of a BLVR with blood procedure (from insertion to withdrawal of the bronchoscope) ranged from 5 to 11 minutes (mean, 8.4 minutes). No complications were seen during the infusion of blood and thrombin.
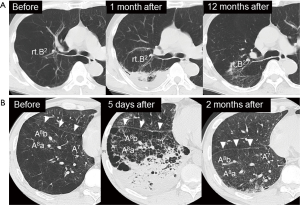
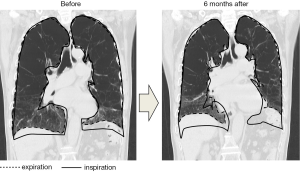
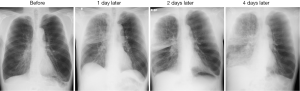
If the infusion was sufficient, 1 to 3 days after the BLVR with blood, transient pneumonia occurred in the lung field of the treated side accompanied by fever, hypoxemia and tachycardia. They were relieved quickly by a single administration of steroid (methylprednisolone 125 mg) with antibacterial therapy (piperacillin/tazobactam 4.5 g ×3 times a day). In addition, eosinophilia was observed for several weeks after the pneumonia was relieved. Although blood was injected into mainly the anterior segment of the upper lobe in all of the procedures, infiltrative shadows were not localized in the upper lobes but extended into the lower lobes in all cases, and the dorsal emphysematous regions of the upper and lower lobes were exposed to exudative fluid which was pulled towards the dorsal lung regions by gravity. No hemoptysis was observed and the contralateral lung did not have infiltrative shadows. To obtain enough infiltration in each procedure, 1 to 4 courses (median value of 2) of the treatment were needed. After recovery of the infiltrative shadows, the dorsal emphysematous area shrank remarkably (Figure 1), and resulted in relief of the patients’ dyspnea.
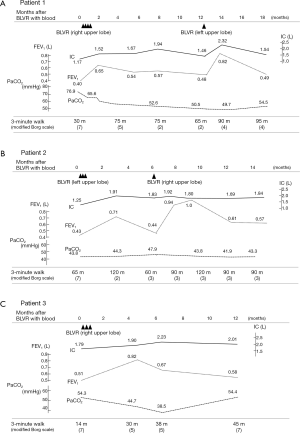
Changes in various indices concerning the pulmonary function of each subject are shown in Figure 2A-C. In patient 1 (Figure 2A), after each procedure of BLVR with blood, improvement of inspiratory capacity was seen from 1.17 L to a maximum of 1.94 L after the first BLVR to the right upper lobe, and from 1.46 to 2.32 L after the second BLVR to the left upper lobe. FEV1 also showed improvement and peaked at 1 to 2 months after each procedure. Patients 2 (Figure 2B) and 3 (Figure 2C) showed the same pattern, and the baseline value and peak value before and after each procedure in the 3 patients are summarized in Table 2. These changes were significant (P=0.015, 0.002).
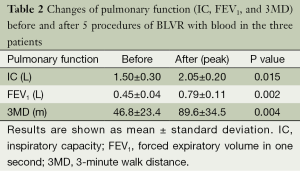
Full table
The improvement of lung function was temporary in all patients, and lung function returned to the baseline level within 6 to 12 months. However, improvements in the 3-minute walk test were maintained for 1 to 6 months longer than the improvement of lung function, and the result of the 3-minute walk test was better at least for 12 months after the procedure compared with that at baseline.
In all cases, shorter recovery time from shortness of breath on exertion facilitated remarkable improvement in activities of daily living such as eating, brushing of teeth, face-washing, walking to the lavatory, and increased frequency of taking a bath.
The SGRQ was administered to patients 2 and 3 at baseline and at 12 months after the procedure and in both patients it improved remarkably; case 2: 67.5 → 51.9 (−15.6 points), case 3: 79.6 → 67.7 (−11.9 points).
Improvements in the range of motion of the diaphragm and doming of the diaphragm were seen in the coronal view of CT image in patient 3 after BLVR with blood (Figure 3).
Discussion
In recent years, less invasive bronchoscopic approaches have been designed to achieve lung volume reduction in patients with severe emphysema.
Currently, the best known devices for BLVR are one-way valves (3,10-15). Other methods include airway bypass (16-18), lung volume reduction coil (19-21), biologic lung volume reduction using fibrin-based glue (8,22-24), and bronchoscopic thermal vapor ablation (25,26). However, most of those methods have issues of cost and heterogeneity of response to the procedures.
In the VENT study, patients with heterogeneous emphysema who received BLVR with Zephyr® endobronchial valve showed a significant improvement in FEV1 and 6 minute walk test compared with the control patients (3). The VENT study showed that heterogeneity of emphysema and fissure completeness in the treated lobe is important for the effectiveness of the therapy. Collateral ventilation can limit the effectiveness of endobronchial valve treatment and is therefore an important predictor of the effectiveness of this therapy (27). The ChartisTM Pulmonary Assessment System (Pulmonx Inc, USA) is commercially available to make assessments regarding the level of collateral ventilation or inter-lobar airflow in the lungs.
Kobayashi and Kanoh (6) performed BLVR with blood in 4 patients with Medical Research Council Dyspnea scale (MRC) grade 2 emphysematous lung disease. The procedures increased FEV1 from 1.10±0.58 to 1.25±0.64 L (values before to 8 weeks after the procedure, mean ± standard deviation) and improved the 6-min walk distance in 2 patients, and no severe complications other than temporary patchy infiltration shadows were observed (6).
In our study, BLVR with blood was performed in patients with MRC grade 4 and resulted in greater improvements in pulmonary function compared to the study of Kobayashi and Kanoh (6). The reason is suggested as follows: our patients had more severe emphysema and more inflated lungs, which induced greater dramatic effects of volume reduction, and although Kobayashi and Kanoh reported only patchy infiltrations after the procedure but no pneumonia, our patients suffered apparent pneumonia caused by autologous blood and thrombin. It is possible that the functional improvement depends on the magnitude of inflammation evoked to some extent.
Initially, we infused 2 mL of autologous blood and 1,000 units of thrombin solution 4 times during one course according to the report of Kobayashi and Kanoh (6), but the inflammation evoked was insufficient. If there was a slight infiltrative shadow, there were few side effects, but there were little improvements in pulmonary function and dyspnea. We therefore increased the dose of blood to 4 mL and that of thrombin to 2,500 units and the number of times to 4-6 times during one course, which induced sufficient inflammation during one course, and there was remarkable improvement of subjective symptoms after relief of the pneumonia. Then, we changed the dosage as described above. Inflammation may induce unexpected serious adverse events especially in patients with very severe COPD, and this method should be performed in carefully monitored settings.
Ingenito et al. (7) reported a study of biological lung volume reduction with instillation of fibrin hydrogel and thrombin into the emphysematous area of a sheep model of emphysema, and found that this procedure promoted fibroblast attachment and collagen synthesis. Their intention was to remodel the hyperinflated emphysematous lung into contracted scar tissue. Kobayashi and Kanoh (6) used autologous blood as the injection material because it has a bioadhesive property and most of all, it is safe and inexpensive. They also reported that they used thrombin to promote clotting of blood in the lung to enhance the lung volume reduction effect.
Although we injected autologous blood into the anterior segment of the right or left upper lobe, the infiltrating shadow extended across the interlobar fissure into the lower lung field (Figure 4). This fact would be explained by the hypothesis that exudative fluid caused by autologous blood and thrombin can easily traverse the lung segment and interlobar fissure via the collateral pathway. Collateral ventilation is highly developed in the lungs of patients with emphysema compared to healthy lungs, and the collateral pathway may traverse lobar fissures in patients with severe emphysema (28-30). This hypothesis is supported by Figure 5; the pulmonary infiltrative shadow seen 4 days after BLVR with blood easily moved by postural change of the patient.
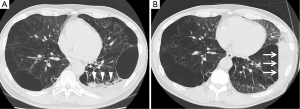
In all cases, after the relief of pneumonia, the emphysematous area that had been exposed to exudative fluid shrank remarkably, while the anterior segment that had not been exposed to exudative fluid showed little volume reduction (Figure 1). These results suggest that it may be important for patients to maintain a posture such that the target lesion is exposed to exudative fluid for several days after the procedure to enhance the effectiveness of the treatment.
In addition, these results suggest that it is difficult to evoke inflammation in a limited area. Nevertheless, the effect of lung volume reduction was obtained without serious adverse events in the patients. One of the reasons may be that the patients in this study had relatively homogeneous emphysema and the area where inflammation spread was all emphysematous area. This fact suggested that it may be better to perform BLVR with blood in patients with homogenous emphysema.
The improvements in lung function, exercise capacity, and PaCO2 after BLVR with blood were considered to be derived from increased ventilation by the increase in diaphragmatic excursion which had been restricted by pulmonary hyperinflation.
Lung functions returned to the baseline status within 6 to 12 months, but the improvement in exercise capacity was maintained for a longer period of time than the improvement in lung function. This discrepancy was also reported in LVRS (31), and rehabilitation is thought to contribute to maintenance of the effect of the volume reduction.
BLVR with blood has several advantages compared with LVRS in that patients do not need to be placed under general anesthesia, the technique is simple, the required time is short, and it allows early restart of rehabilitation. Compared with other BLVR methods, this method is less expensive and physicians do not need to consider complications caused by placement of a foreign body intrabronchially for a long period of time.
Candidates for BLVR with blood are thought to include patients who have lung hyperinflation, low exercise capacity, and unrelieved dyspnea despite appropriate medical therapy, and who can continue rehabilitation. Meanwhile, patients with homogeneous emphysema, %FEV1 less than 20% and/or PCO2 greater than 60 torr are not eligible for LVRS, but may be eligible for BLVR with blood if bronchoscopy is feasible.
As to long-term results, at 1.5 years later, the exercise capacity of our patients was better than that at baseline and no severe complications were seen.
Therefore, BLVR with blood is thought to be effective as a palliative therapy for patients with severe COPD. However, there are still problems in that the improvements of lung function and exercise capacity are temporary, and there is a risk of acute exacerbation of COPD induced by pneumonia caused by the procedure. Also, this procedure was carried out in a small number of patients and the safety and effectiveness of BLVR with blood were not established in contrast to BLVR with endobronchial valves. Therefore, further studies are needed to evaluate the efficacy and safety of this procedure.
Conclusions
The results of this study suggest that BLVR with blood can improve pulmonary function, exercise tolerance, and quality of life and may produce benefits as a palliative treatment in patients with very severe COPD.
Acknowledgements
The authors are indebted to Professor Yukihiko Sugiyama in the Department of Respiratory Medicine, Jichi Medical University, for his valuable advice.
Disclosure: The authors declare no conflict of interest.
References
- Fishman A, Fessler H, Martinez F, et al. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075-83. [PubMed]
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [PubMed]
- Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233-44. [PubMed]
- Kanoh S, Kobayashi H, Motoyoshi K. Intrabullous blood injection for lung volume reduction. Thorax 2008;63:564-5. [PubMed]
- Kanoh S, Kobayashi H, Motoyoshi K. Bronchoscopic blood injection reducing lung volume in lymphangioleiomyomatosis. Ann Thorac Surg 2009;87:1266-8. [PubMed]
- Kobayashi H, Kanoh S. Bronchoscopic autologous blood injection for lung volume reduction Nihon Kokyuki Gakkai Zasshi 2009;47:765-71. [PubMed]
- Ingenito EP, Berger RL, Henderson AC, et al. Bronchoscopic lung volume reduction using tissue engineering principles. Am J Respir Crit Care Med 2003;167:771-8. [PubMed]
- Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992;145:1321-7. [PubMed]
- Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992;145:1321-7. [PubMed]
- Wood DE, McKenna RJ Jr, Yusen RD, et al. A multicenter trial of an intrabronchial valve for treatment of severe emphysema. J Thorac Cardiovasc Surg 2007;133:65-73. [PubMed]
- Sterman DH, Mehta AC, Wood DE, et al. A multicenter pilot study of a bronchial valve for the treatment of severe emphysema. Respiration 2010;79:222-33. [PubMed]
- Hopkinson NS, Toma TP, Hansell DM, et al. Effect of bronchoscopic lung volume reduction on dynamic hyperinflation and exercise in emphysema. Am J Respir Crit Care Med 2005;171:453-60. [PubMed]
- Wan IY, Toma TP, Geddes DM, et al. Bronchoscopic lung volume reduction for end-stage emphysema: report on the first 98 patients. Chest 2006;129:518-26. [PubMed]
- Strange C, Herth FJ, Kovitz KL, et al. Design of the Endobronchial Valve for Emphysema Palliation Trial (VENT): a non-surgical method of lung volume reduction. BMC Pulm Med 2007;7:10. [PubMed]
- Ninane V, Geltner C, Bezzi M, et al. Multicentre European study for the treatment of advanced emphysema with bronchial valves. Eur Respir J 2012;39:1319-25. [PubMed]
- Lausberg HF, Chino K, Patterson GA, et al. Bronchial fenestration improves expiratory flow in emphysematous human lungs. Ann Thorac Surg 2003;75:393-7; discussion 398. [PubMed]
- Choong CK, Macklem PT, Pierce JA, et al. Airway bypass improves the mechanical properties of explanted emphysematous lungs. Am J Respir Crit Care Med 2008;178:902-5. [PubMed]
- Cardoso PF, Snell GI, Hopkins P, et al. Clinical application of airway bypass with paclitaxel-eluting stents: early results. J Thorac Cardiovasc Surg 2007;134:974-81. [PubMed]
- Slebos DJ, Klooster K, Ernst A, et al. Bronchoscopic lung volume reduction coil treatment of patients with severe heterogeneous emphysema. Chest 2012;142:574-82. [PubMed]
- Shah PL, Zoumot Z, Singh S, et al. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. Lancet Respir Med 2013;1:233-40. [PubMed]
- Herth FJ, Eberhard R, Gompelmann D, et al. Bronchoscopic lung volume reduction with a dedicated coil: a clinical pilot study. Ther Adv Respir Dis 2010;4:225-31. [PubMed]
- Criner GJ, Pinto-Plata V, Strange C, et al. Biologic lung volume reduction in advanced upper lobe emphysema: phase 2 results. Am J Respir Crit Care Med 2009;179:791-8. [PubMed]
- Herth FJ, Gompelmann D, Stanzel F, et al. Treatment of advanced emphysema with emphysematous lung sealant (AeriSeal®).Treatment of advanced emphysema with emphysematous lung sealant (AeriSeal®). Respiration 2011;82:36-45. [PubMed]
- Refaely Y, Dransfield M, Kramer MR, et al. Biologic lung volume reduction therapy for advanced homogeneous emphysema. Eur Respir J 2010;36:20-7. [PubMed]
- Snell GI, Hopkins P, Westall G, et al. A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Ann Thorac Surg 2009;88:1993-8. [PubMed]
- Gompelmann D, Eberhardt R, Ernst A, et al. The localized inflammatory response to bronchoscopic thermal vapor ablation. Respiration 2013;86:324-31. [PubMed]
- Gompelmann D, Eberhardt R, Michaud G, et al. Predicting atelectasis by assessment of collateral ventilation prior to endobronchial lung volume reduction: a feasibility study. Respiration 2010;80:419-25. [PubMed]
- Morrell NW, Wignall BK, Biggs T, et al. Collateral ventilation and gas exchange in emphysema. Am J Respir Crit Care Med 1994;150:635-41. [PubMed]
- Cetti EJ, Moore AJ, Geddes DM. Collateral ventilation. Thorax 2006;61:371-3. [PubMed]
- Aljuri N, Freitag L. Validation and pilot clinical study of a new bronchoscopic method to measure collateral ventilation before endobronchial lung volume reduction. J Appl Physiol (1985) 2009;106:774-83. [PubMed]
- Flaherty KR, Kazerooni EA, Curtis JL, et al. Short-term and long-term outcomes after bilateral lung volume reduction surgery: prediction by quantitative CT. Chest 2001;119:1337-46. [PubMed]


