Diagnostic performance of cardiac magnetic resonance for the detection of acute cardiac allograft rejection: a systematic review and meta-analysis
Introduction
Currently, heart transplantation (HTX) is the only definitive treatment for end-stage heart failure. Despite advances in immunosuppressive therapy, acute cardiac allograft rejection (ACAR) remains the most common concerns during the first year after transplantation. Approximately 40% patients will experience at least one episode of ACAR within this period (1). Moreover, ACAR is responsible for approximately 12% of mortality between 1 and 12 months of post-transplantation, and an independent risk factor for developing into cardiac allograft vasculopathy (CAV). Even with effective treatments, an episode of ACAR occurring in the first year will increase two-year and four-year fatalities (2). Therefore, early detecting and curbing ACAR is crucial to the survival of transplant recipients.
However, clinical features of ACAR are not reliable, with patients usually remaining asymptomatic until hemodynamic compromise occurs. Invasive surveillance procedure is mandatory to perform routinely and frequently in order to detect ACAR, and hence augment immunosuppressive therapy at an earlier stage, with the aim of preventing progression to more severe rejection, and achieving better long-term outcome. Right ventricular endomyocardial biopsy (EMB) still represents the clinical gold standard in monitoring cardiac allograft rejection. Nevertheless, this invasive diagnostic procedure is concomitant with several, albeit rare, major complications such as cardiac tamponade and permanent heart block. Deckers et al. reported a 6% overall complication rate in a prospective study of 546 EMB (3). A higher global complication rate described by Hosenpud group was 14% (4). Felker et al. and Frustaci et al. demonstrated cardiac tamponade rates were 0.31% and 0.27%, respectively (5,6). The incidence of permanent atrioventricular block ranged from 0.04-1.7% (7,8). EMB also has a number of limitations like exposure to radiation, sample error, myocardial scarring and venous thrombosis (9,10). Non-invasive but equally accurate technique to detect rejection in cardiac transplant patients is highly desirable.
Many promising imaging techniques have been tried to develop a sensitive and specific non-invasive method. Of the many diagnostic techniques, only echocardiography and cardiac magnetic resonance (CMR) imaging have demonstrated a strong correlation with EMB (11).
CMB, a diagnostic modality is considered as the gold standard to evaluate cardiac morphology, ventricular function, myocardial perfusion and viability (12). Several studies have addressed the diagnostic accuracy of CMR to assess the rejection grade of ACAR compared with EMB. But the methodological heterogeneity, such as different parameters and cut-off value, which led to conflicting outcomes among individual studies, limited the clinical application of CMR. It is necessary to further assess the diagnostic value of CMR for the detection of ACAR. Accordingly, we seek a comprehensive, systematic literature review and meta-analysis for the purpose.
Materials and methods
Data resource and search strategy
We systematically searched the Cochrance clinical trials database, Medline/Pubmed and EMBASE to identify eligible studies prior to September 1, 2014. No starting date was limited. In addition to database searches, we reviewed the references of included studies and other relevant review articles to obtain a comprehensive list of included studies. Two authors (Wei Lu and Jun Zheng) searched and reviewed database independently. Disagreements were resolved by discussion or upon consensus with a third reviewer. We used the following medical subject headings and search terms: “magnetic resonance imaging” “cardiac magnetic resonance”, “heart transplantation” and “graft rejection”. Searching formula is shown in Supplement material.
Study selection
Selection criteria: (I) type of study: diagnostic accuracy test; (II) population: underwent HTX with all age spectrums; (III) index test: CMR; (IV) reference standard: EMB; (V) language: published in English.
Exclusion criteria: (I) type of study: reviews, case reports, editorial, presentations or animal researches; (II) sample size <10 patients; (III) true-positive (TP), false-positive (FP), true-negative (TN) and false negative (FN) data were unavailable or could not be derived from articles.
Data extraction and quality assessment
The following variables were extracted from each study: author, publication year, country, demographic characteristics of study population, study design (prospective or retrospective), recruitment method (consecutive or random), interval between HTX and CMR, interval between CMR and EMB, blind, CMR parameter, cut-off value, rejection grade of detection, reference of histological interpretation for rejection grade, and number of TP, FP, TN and FN. If studies enrolled all of subjects during a certain period, and conduced CMR and EMB on them, the recruitment method will be defined as “consecutive”, even if the studies did not describe the method. Two authors extracted data from eligible studies independently (Wei Lu and Xu-Dong Pan). The methodological quality of eligible studies was assessed by two authors (Ming-Duo Zhang and Tie-Yuan Zhu) independently using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2), an assessment tool used in systematic reviews to evaluate the risk of bias and applicability of primary diagnostic accuracy studies (13). In the same way, disagreements were resolved by discussing together or appealing to a third author
Data synthesis and statistical analysis
Meta-DiSc version 1.4 (14) statistical software was used for our study. Analysis process included four steps as follows. First of all, Spearman correlation coefficient between sensitivity (se) and specificity (sp), and P value, were computed to explore heterogeneity arising from a threshold effect. Subgroup analysis was conducted according to different threshold variables. Secondly, non-threshold heterogeneity was explored by using inconsistency (I2) value and χ2 test (15). I2 value within 25-49%, 50-74% or 75-100% was considered a low, moderate or high degree of heterogeneity respectively (16). Subsequently, sensitivity analysis was applied to explore the source in case of the existence of non-threshold heterogeneity, and DerSimonian-Laird random effects model was considered if necessary (17). Otherwise, pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the curve (AUC) with 95% confidence interval (CI) were calculated by using Mantel-Haenzsel fixed effects models (8). The pooled DOR was used for constructing summary receiver operating characteristic curve (SROC), with its Q point representing the maximal joint of sensitivity and specificity (18,19).
Results
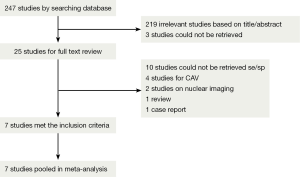
Database search and additional citation tracking of review and original articles produced 247 potentially relevant citations, 92 from Medline/Pubmed, 155 from EMBASE and zero from Cochrane library. After getting rid of ineligible articles, such as duplicated articles, case reports, reviews or animal researches, we submitted 25 studies for a full text review. A total of 18 articles were excluded due to unavailable data or detection for CAV. Finally, seven eligible studies were included in our meta-analysis. Detailed process is presented in Figure 1.
Characteristics and quality of included articles
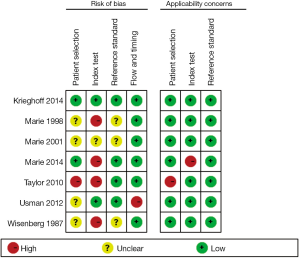
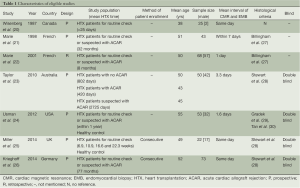
Seven included articles were published during a long span of time, from 1987 to 2014 (20-26). Prospective studies account for 85.7% (6/7) of all eligible studies. A total of 334 patients and 802 CMR/EMB results were included in the analysis. Characteristics of included studies are shown in Table 1. Of all the studies, two studies consecutively recruited subjects for research (25,26). There are four studies that complied with double-blind principle when interpreting index test and reference standards. The rest of three articles did not mention whether a blind method was conducted. One study described EMB was performed within 1 week of CMR and without any therapeutic change between the two investigations (21). Three studies performed EMB and CMR on the same day. EMB was used as a reference standard in all eligible studies. However, one study included an additionally clinical reference standard that patients presenting hemodynamic compromise, even with a negative histological result, were deemed to have ACAR (24). Three studies performed CMR using a pre-specified threshold value (22,24,26). There were two studies enrolling healthy control to determine a baseline for CMR parameters (24,25). The risk of bias and applicability of the studies was evaluated based on QUADAS-2 shown in Figures S1,S2.
Full table
Diagnostic information and accuracy
The results of the diagnostic accuracy test of CMR for ACAR in each study are shown in Table 2. Five parameters of CMR [e.g., T2 relaxation time, T2 short time inversion recovery (STIR) intensity, T1 myocardial contrast enhancement, late gadolinium enhancement (LGE) and peak systolic circumferential strain] were applied to detect moderate ACAR (rejection grade ≥2). One study (26) provided sensitivities and specificities for detection of both ACAR grade ≥2 and ≥1B, but only three patients diagnosed as histological grade ≥2 were recorded. Six studies used T2 parameter, such as T2 relaxation time and T2 STIR value, which are associated with myocardial edema, to detect ACAR. Two studies employed T1 myocardial contrast enhancement with intravenous administration of gadobutrol to assess rejection grade (23,26). Cardiac functional parameter, peak systolic circumferential strain, was only performed in one study (25). Two studies combined two parameters to achieve more accurate results (23,26).
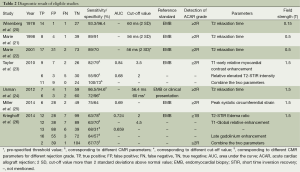
Full table
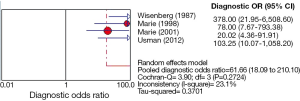
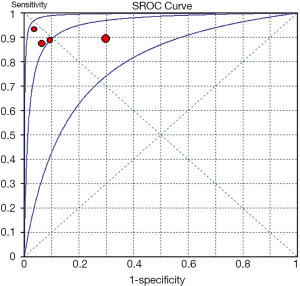
Of all the studies, the most widely used parameter was T2 value related to myocardial edema. We planned to perform a meta-analysis among seven individual studies. However, only one study, including three positive results was ruled out of meta-analysis (26). Unfortunately, only four studies (20-22,24) using T2 relaxation time were finally included in the meta-analysis, because the number of eligible studies using the other parameters is less than three. The Spearman correlation coefficient was computed as a result of –0.200 with a P value 0.800, which suggested the absence of a threshold effect. I2 value of sensitivity, NLR and DOR were 0.0%, 0.0% and 23.1%, respectively, and corresponding P value of χ2 test were 0.965, 0.929 and 0.272 respectively. These results indicated the absence of heterogeneity. However, specificity and PLR presented a high degree of heterogeneity with I2 value of 87.4% and 85.9%, respectively. One of common approaches to examine sources of heterogeneity is sensitivity analysis, where one study is excluded at a time and the impact of removing each of the studies is evaluated on the summary results (31). In our study, we applied sensitivity analysis to explore the source of heterogeneity. The study of Wisenberg et al. (20), which applied a relatively lower field strength and a higher cutoff value compared to other studies, was considered to be removed for testing its effects on the final result. Yet, the new data still showed a high degree of heterogeneity in specificity and PLR. Homogeneity can be achieved only if the study of Marie et al. (22) was excluded. We comprehensively analyzed all characteristics of the study, but were unable to trace the origin of heterogeneity on the basis of current data. Consequently, we employed a DerSimonian-Laird random effects model to pool the indices. The details of pooled results and SROC curve were demonstrated in Figures 2,3,4,5.


Discussion
EMB remains the gold standard method for ACAR surveillance. Due to sampling error associated with the inhomogeneous nature of ACAR, histological “false negative” ACAR is reported to occur in up to 20% of patients (32). Furthermore, EMB is an invasive, expensive and uncomfortable procedure to patients. These drawbacks prevent more frequent monitoring and, thus, limit optimal immunosuppressive therapy in time. Despite many imaging modalities, such as echocardiography, magnetic resonance imaging and positron emission tomography, have been developed, noninvasive detection of ACAR remains a clinical challenge. Echocardiography is one of the most ubiquitous tools for monitoring ACAR since it is easily performable and time saving. Its versatility allows it to be applied in a wide variety of circumstances during the post-transplant period. The indices of echocardiography, such as left ventricular size, wall thickness, mass, pericardial effusion and ejection fraction, are insensitive markers of ACAR (33). Doppler indices of mitral valve inflow are the most widely investigated parameter for detecting ACAR. However, none of studies have shown sufficient accuracy for clinical adoption, because many factors, such as age, heart rate and loading conditions affect the parameters significantly (34). Ciliberto et al. performed a study including 130 patients to explore the diagnostic value of two echocardiographic parameters, and found pressure half time and isovolumetric relaxation time showed a rather poor sensitivity on detecting ACAR (35). Dandel et al. using tissue dopplor parameters, like peak systolic wall motion velocity and diastolic wall motion velocity, presented very high sensitivities and specificities for ACAR in a studiy of 293 patients (36). However, Palka et al. reported low sensitivities and specificities by employing the similar parameters (37). Echocardiography is highly operator-dependent imaging modality that may produce an obscure results in detecting ACAR.
Therefore, in comparison, CMR can be considered as a potential modality to improve the diagnostic accuracy for ACAR. CMR is the gold standard modality for evaluation of ventricular morphology, volume, function and mass due to superior image quality and tissue resolution as compared to echocardiography and nuclear modality (38). The meta-analysis revealed T2 relaxation time is the most widely used parameter to detect ACAR grade ≥2. T2 relaxation occurs due to the interaction between hydrogen nuclei and its exponential decay time-constant. This parameter is directly proportional to myocardial water content (39). Multiple studies using animal transplant models have presented a significant positive correlation between T2 relaxation time and histological severity of ACAR, and ex vivo myocardial water content (40-42). Furthermore, T2 relaxation time appears to be abolished with immunosuppressive therapy (43-45). Except for the seven studies included in the meta-analysis, there were another three human trials comparing T2 relaxation time to ACAR as determined by EMB. One showed a significant correlation between T2 relaxation time and ACAR (46), and the other two studies that did not find a correlation both gated their image acquisition to ventricular systole which often leads to signal loss and poor image quality (47,48).
The meta-analysis of T2 relaxation time showed summary sensitivity and specificity of 90% (95% CI, 79-97%) and 83% (95% CI, 78-88%) respectively. Moreover, the summary PLR and NLR was 8.75 (95% CI, 2.66-28.78) and 0.12 (95% CI, 0.05-0.28) respectively. DOR is a single indicator of test accuracy that combines the sensitivity and specificity data into a single number. The SROC curve presents a global summary of test performance and shows the tradeoff between sensitivity and specificity. The summary DOR, the AUC of the SROC and Q index were 61.66 (95% CI, 18.09-210.10), 0.954 and 0.897, respectively, which showed a relatively high accuracy, and that CMR parameter, T2 relaxation time, is helpful in the detection of ACAR. However, the existence of high degree heterogeneity in specificity and PLR, the source of which was unable to be explored, limits the diagnostic application of T2 relaxation time. Moreover, the specificity of T2 relaxation time was related not only to methodological concerns but to the prevalence of ACAR after HTX. ISHLT Guildlines for the care of heart transplants recipients [2010] reported ACAR is the most common complication in the first 6 months. The incidence of ACAR ranged from 20-40% in the first postoperative year (49). Other myopathies like myocardial infarction and myocarditis may also induce a rise in T2 signal secondary to myocardial edema, although they rarely occurred within the first year of HTX (50,51). Accordingly, the value of T2 relaxation time should be comprehensively analyzed. The optimal cut-off value to detect ACAR is T2 relaxation time more than two standard deviations (SD) above the normal value, about 56-60 ms on the basis of different study conditions, which was used in the four included studies. Considering the limitation of sample size, only four studies enrolling 196 patients, the optimal cutoff value still needs further inspection.
Except for T2 relaxation time, four parameters (i.e., T2-STIR intensity, T1-early relative myocardial contrast enhancement, LGE, and peak systolic circumferential strain), were applied for evaluation of ACAR. The diagnostic accuracy could not be summarized on these parameters because of the small number of studies. T2-STIR intensity is influenced by myocardial water content and can clinically assess myocardial inflammation (52). Yet, the diagnostic performance of T2-STIR intensity for ACAR has been inconsistent in literatures which has shown mixed results (53,54). T1-early contrast enhancement is conducted by injecting intravenous gadolinium and acquiring enhanced T1 signal early after contrast administration. The signal intensity, in proportion to the degree of myocardial perfusion, reflects hyperemia in inflammatory tissue (55). However, previous human trials showed myocardial contrast enhancement was not able to consistently identify ACAR. Alemnar et al. tested several variables of contrast enhanced signal intensity and found no association with rejection (54). Mousseaux et al. found an increase in myocardial enhancement in rejected patients compared with non-rejected patients, but myocardial enhancement could not distinguish rejection grade (47). LGE can be used in CMR to detect myocardial scar or fibrosis. Similarly, several studies found the parameters of ventricular systolic function as measured by CMR are associated with rejection, but these variables are probably of insufficient sensitivity to discriminate different rejection grade (56-58). Until now, no studies have revealed a correlation between LGE and ACAR.
In our meta-analysis, Taylor et al. and Krieghoff et al. employed two parameters, T2-STIR intensity and T1-early relative myocardial contrast enhancement, to detect ACAR (23,26). In particular, they combined two parameters and applied the similar cut-off value that significantly improved the diagnostic value on ruling out therapeutically relevant ACAR with a higher sensitivity and negative predictive value (NPV) comparing to single parameter test. In view of invasive and “false-negative” nature of EMB, multi-parameter CMR may have the potential of non-invasive tool for the exclusion of all ACAR. Moreover, ISHLT Guildlines for the care of heart transplants recipients [2010] summarized several noninvasive methods for ACAR and highlighted several studies have identified a strong correlation between plasma biomarkers and ACAR (49). These studies have shown B-type natriuretic peptide levels (BNP) and troponin T (TnT) levels have excellent NPVs, from 95% to 97.3%, in excluding severe rejection (59-62). If multi-parameter CMR was combined with these biomarkers, this modality, in theory, might rule out of all negative results. Hofmann et al. found high sensitive TnT (hs TnT) and LGE of CMR provided complementary value on diagnosing CAV. Low hs TnT combined with high CMR value provided a nearly 100% of NPV for adverse cardiac events (63). However, few studies focus on the respect for ACAR so far, and further researches aiming at multi-modality including imaging, plasma biomarkers or electrophysiology may be desired.
Myocardial strain describes the change in myocardial deformation and has been found to reflect myocardial contractility best, while strain parameters are pre-load and after-load dependent and may change with ventricular dimensions (64). Its high sensitivity for subtle deteriorations of myocardial function makes strain a promising parameter in the detection of early disease stages, when global functional parameters may still be normal. Only one eligible study used peak systolic circumferential strain to monitor ACAR, and its diagnostic accuracy did not surpass other parameters (25). However, Korosoglou et al. demonstrated a promising performance of strain rate for screening chronic rejection and cardiac vasculopathy with stenosis ≥50% (65). They achieved 100% sensitivity and 100% NPV when the cut-off value of mean diastolic strain rate was set at 43/second. Further researches may supply a comprehensive assessment on the diagnostic value of myocardial strain for ACAR.
In addition, Krieghoff et al. is the only study using CMR for detection of sub-clinical ACAR (rejection grade ≥1B) (26). Because parts of sub-clinical ACAR have the potential to progress into severe rejection, and grade 1B have been combined into grade 2R in the revision of ISHLT, multi-sequence CMR might be considered as an alternative modality for surveillance sub-clinical ACAR (49). However, these combined parameters were not evaluated comprehensively by meta-analysis. For rather poor specificity and PPV, and a small number of studies, the diagnostic performance for sub-clinical ACAR is still limited. Further studies are required to confirm their diagnostic value.
Limitations
Similar to other diagnostic meta-analysis, several limitations exist exactly in our study. First, studies ranged from 1987 to 2014, hence results may be affected by the progression of technique and device update. Second, T2 relaxation time is the most widely used index, but only four studies applied the index in the meta-analysis. Because the number of eligible studies including other CMR indices is less than three, hence, we cannot comprehensively evaluate the diagnostic performance of CMR. Third, the presence of high degree heterogeneity in specificity and PLR may have overestimated or underestimated the actual diagnostic accuracy. Moreover, two eligible studies did not mention double-blind principle, thus, it might increase the possibility of review bias; only two studies were confirmed to have enrolled patients consecutively that might cause selection bias; patients with contraindication of CMR were excluded from researches might also generate selection bias; all of eligible study published in English that could result in publication bias. Finally, the sample size of meta-analysis is rather small, only including four studies with 196 patients. A larger sample size could acquire more reliable results.
Conclusions
Although the existence of limitations, to our knowledge, this is the first meta-analysis to explore the value of CMR in the diagnosis of ACAR. The meta-analysis and systematic review demonstrate that CMR seems to have a high sensitivity and moderate specificity in the diagnosis of ACAR. However, a result of CMR for diagnostic ACAR should be comprehensively considered by physicians and imaging experts in the context of clinical presentations and imaging feature. Further investigations are still required to test different parameters and study condition.
Acknowledgements
We would like to thank the authors of the original studies included in this meta-analysis.
Disclosure: The authors declare no conflict of interest.
Supplement material
Medline search formula: ((((“Magnetic Resonance Imaging”[Mesh]) OR ((magnetic AND resonance AND imaging) OR MRI OR MR OR CMR OR (magnetic AND resonance)))) AND ((“Graft Rejection”[Mesh]) OR ((transplantation* OR grafting* OR graft* OR allograft*) AND rejection*))) AND ((“Heart Transplantation”[Mesh]) OR ((Heart OR Cardiac) AND (transplantation* OR grafting* OR graft* OR allograft*))).
Embase search formula: ‘mri’ OR ‘mri’/exp OR mri OR (magnetic AND resonance) OR (magnetic AND resonance AND (‘imaging’ OR ‘imaging’/exp OR imaging)) OR ‘mr’ OR ‘mr’/exp OR mr OR cmr AND (‘heart’ OR ‘heart’/exp OR heart OR cardiac) AND (‘transplantation’ OR ‘transplantation’/exp OR transplantation OR transplanted OR transplant OR ‘allograft’ OR ‘allograft’/exp OR allograft) AND (rejection OR reject) AND ([article]/lim OR [article in press]/lim OR [review]/lim) AND ([article]/lim OR [article in press]/lim) AND [english]/lim AND [humans]/lim AND [embase]/lim.
References
- Patel JK, Kobashigawa JA. Should we be doing routine biopsy after heart transplantation in a new era of anti-rejection? Curr Opin Cardiol 2006;21:127-31. [PubMed]
- Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report--2010. J Heart Lung Transplant 2010;29:1089-103. [PubMed]
- Deckers JW, Hare JM, Baughman KL. Complications of transvenous right ventricular endomyocardial biopsy in adult patients with cardiomyopathy: a seven-year survey of 546 consecutive diagnostic procedures in a tertiary referral center. J Am Coll Cardiol 1992;19:43-7. [PubMed]
- Hosenpud JD. Complications of endomyocardial biopsy. In: Karson J, Morton MS. eds. Complications of Cardiac Catheterization and Angiography. Prevention and Management. Mt Kisco: Futura, 1989:135-54.
- Felker GM, Hu W, Hare JM, et al. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine (Baltimore) 1999;78:270-83. [PubMed]
- Frustaci A, Pieroni M, Chimenti C.. The role of endomyocardial biopsy in the diagnosis of cardiomyopathies. Ital Heart J 2002;3:348-53. [PubMed]
- Holzmann M, Nicko A, Kühl U, et al. Complication rate of right ventricular endomyocardial biopsy via the femoral approach: a retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation 2008;118:1722-8. [PubMed]
- Jang SY, Cho Y, Song JH, et al. Complication rate of transfemoral endomyocardial biopsy with fluoroscopic and two-dimensional echocardiographic guidance: a 10-year experience of 228 consecutive procedures. J Korean Med Sci 2013;28:1323-8. [PubMed]
- Baraldi-Junkins C, Levin HR, Kasper EK, et al. Complications of endomyocardial biopsy in heart transplant patients. J Heart Lung Transplant 1993;12:63-7. [PubMed]
- Spiegelhalter DJ, Stovin PG. An analysis of repeated biopsies following cardiac transplantation. Stat Med 1983;2:33-40. [PubMed]
- Dodd DA, Brady LD, Carden KA, et al. Pattern of echocardiographic abnormalities with acute cardiac allograft rejection in adults: correlation with endomyocardial biopsy. J Heart Lung Transplant 1993;12:1009-17. [PubMed]
- Constantine G, Shan K, Flamm SD, et al. Role of MRI in clinical cardiology. Lancet 2004;363:2162-71. [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [PubMed]
- Zamora J, Abraira V, Muriel A, et al. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6:31. [PubMed]
- Lijmer JG, Bossuyt PM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med 2002;21:1525-37. [PubMed]
- Ferreira ML, Smeets RJ, Kamper SJ, et al. Can we explain heterogeneity among randomized clinical trials of exercise for chronic back pain? A meta-regression analysis of randomized controlled trials. Phys Ther 2010;90:1383-403. [PubMed]
- Copas J, Shi JQ. Meta-analysis, funnel plots and sensitivity analysis. Biostatistics 2000;1:247-62. [PubMed]
- Irwig L, Macaskill P, Glasziou P, et al. Meta-analytic methods for diagnostic test accuracy. J Clin Epidemiol 1995;48:119-30; discussion 131-2. [PubMed]
- Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 1993;12:1293-316. [PubMed]
- Wisenberg G, Pflugfelder PW, Kostuk WJ, et al. Diagnostic applicability of magnetic resonance imaging in assessing human cardiac allograft rejection. Am J Cardiol 1987;60:130-6. [PubMed]
- Marie PY, Carteaux JP, Angioï M, et al. Detection and prediction of acute heart transplant rejection: preliminary results on the clinical use of a "black blood" magnetic resonance imaging sequence. Transplant Proc 1998;30:1933-5. [PubMed]
- Marie PY, Angioï M, Carteaux JP, et al. Detection and prediction of acute heart transplant rejection with the myocardial T2 determination provided by a black-blood magnetic resonance imaging sequence. J Am Coll Cardiol 2001;37:825-31. [PubMed]
- Taylor AJ, Vaddadi G, Pfluger H, et al. Diagnostic performance of multisequential cardiac magnetic resonance imaging in acute cardiac allograft rejection. Eur J Heart Fail 2010;12:45-51. [PubMed]
- Usman AA, Taimen K, Wasielewski M, et al. Cardiac magnetic resonance T2 mapping in the monitoring and follow-up of acute cardiac transplant rejection: a pilot study. Circ Cardiovasc Imaging 2012;5:782-90. [PubMed]
- Miller CA, Naish JH, Shaw SM, et al. Multiparametric cardiovascular magnetic resonance surveillance of acute cardiac allograft rejection and characterisation of transplantation-associated myocardial injury: a pilot study. J Cardiovasc Magn Reson 2014;16:52. [PubMed]
- Krieghoff C, Barten MJ, Hildebrand L, et al. Assessment of sub-clinical acute cellular rejection after heart transplantation: comparison of cardiac magnetic resonance imaging and endomyocardial biopsy. Eur Radiol 2014;24:2360-71. [PubMed]
- Billingham ME, Cary NR, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant 1990;9:587-93. [PubMed]
- Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005;24:1710-20. [PubMed]
- Gradek WQ, D'Amico C, Smith AL, et al. Routine surveillance endomyocardial biopsy continues to detect significant rejection late after heart transplantation. J Heart Lung Transplant 2001;20:497-502. [PubMed]
- Tan CD, Baldwin WM 3rd, Rodriguez ER. Update on cardiac transplantation pathology. Arch Pathol Lab Med 2007;131:1169-91. [PubMed]
- Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol 2008;37:1148-57. [PubMed]
- Tang Z, Kobashigawa J, Rafiei M, et al. The natural history of biopsy-negative rejection after heart transplantation. J Transplant 2013;2013:236720.
- Knosalla C, Hummel M, Müller J, et al. Diagnosis of heart graft rejection. Curr Opin Organ Transplant 2000;5:118-25.
- Mena C, Wencker D, Krumholz HM, et al. Detection of heart transplant rejection in adults by echocardiographic diastolic indices: a systematic review of the literature. J Am Soc Echocardiogr 2006;19:1295-300. [PubMed]
- Ciliberto GR, Mascarello M, Gronda E, et al. Acute rejection after heart transplantation: noninvasive echocardiographic evaluation. J Am Coll Cardiol 1994;23:1156-61. [PubMed]
- Dandel M, Hummel M, Müller J, et al. Reliability of tissue Doppler wall motion monitoring after heart transplantation for replacement of invasive routine screenings by optimally timed cardiac biopsies and catheterizations. Circulation 2001;104:I184-91. [PubMed]
- Palka P, Lange A, Galbraith A, et al. The role of left and right ventricular early diastolic Doppler tissue echocardiographic indices in the evaluation of acute rejection in orthotopic heart transplant. J Am Soc Echocardiogr 2005;18:107-15. [PubMed]
- La Gerche A, Claessen G, Van de Bruaene A, et al. Response to letter regarding article, "Cardiac magnetic resonance imaging: a new gold standard for ventricular volume quantification during high-intensity exercise Circ Cardiovasc Imaging 2013;6:e20. [PubMed]
- Higgins CB, Herfkens R, Lipton MJ, et al. Nuclear magnetic resonance imaging of acute myocardial infarction in dogs: alterations in magnetic relaxation times. Am J Cardiol 1983;52:184-8. [PubMed]
- Sasaguri S, LaRaia PJ, Fabri BM, et al. Early detection of cardiac allograft rejection with proton nuclear magnetic resonance. Circulation 1985;72:II231-6. [PubMed]
- Aherne T, Tscholakoff D, Finkbeiner W, et al. Magnetic resonance imaging of cardiac transplants: the evaluation of rejection of cardiac allografts with and without immunosuppression. Circulation 1986;74:145-56. [PubMed]
- Huber DJ, Kirkman RL, Kupiec-Weglinski JW, et al. The detection of cardiac allograft rejection by alterations in proton NMR relaxation times. Invest Radiol 1985;20:796-802. [PubMed]
- Kurland RJ, West J, Kelley S, et al. Magnetic resonance imaging to detect heart transplant rejection: sensitivity and specificity. Transplant Proc 1989;21:2537-43. [PubMed]
- Sasaki H, Sada M, Nishimura T, et al. The expanded scope of effectiveness of nuclear magnetic resonance imaging to determine cardiac allograft rejection. Transplant Proc 1987;19:1062-4. [PubMed]
- Tscholakoff D, Aherne T, Yee ES, et al. Cardiac transplantations in dogs: evaluation with MR. Radiology 1985;157:697-702. [PubMed]
- Lund G, Morin RL, Olivari MT, et al. Serial myocardial T2 relaxation time measurements in normal subjects and heart transplant recipients. J Heart Transplant 1988;7:274-9. [PubMed]
- Mousseaux E, Farge D, Guillemain R, et al. Assessing human cardiac allograft rejection using MRI with Gd-DOTA. J Comput Assist Tomogr 1993;17:237-44. [PubMed]
- Doornbos J, Verwey H, Essed CE, et al. MR imaging in assessment of cardiac transplant rejection in humans. J Comput Assist Tomogr 1990;14:77-81. [PubMed]
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30:2493-537. [PubMed]
- Peter S, Hulme O, Deuse T, et al. ST-elevation myocardial infarction following heart transplantation as an unusual presentation of coronary allograft vasculopathy: a case report. Transplant Proc 2013;45:787-91. [PubMed]
- Loria K, Jessurun J, Shumway SJ, et al. Early recurrence of chronic active myocarditis after heart transplantation. Hum Pathol 1994;25:323-6. [PubMed]
- Abdel-Aty H, Boyé P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol 2005;45:1815-22. [PubMed]
- Smart FW, Young JB, Weilbaecher D, et al. Magnetic resonance imaging for assessment of tissue rejection after heterotopic heart transplantation. J Heart Lung Transplant 1993;12:403-10. [PubMed]
- Almenar L, Igual B, Martínez-Dolz L, et al. Utility of cardiac magnetic resonance imaging for the diagnosis of heart transplant rejection. Transplant Proc 2003;35:1962-4. [PubMed]
- Abdel-Aty H, Boyé P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol 2005;45:1815-22. [PubMed]
- Ciliberto GR, Mascarello M, Gronda E, et al. Acute rejection after heart transplantation: noninvasive echocardiographic evaluation. J Am Coll Cardiol 1994;23:1156-61. [PubMed]
- McNamara D, Di Salvo T, Mathier M, et al. Left ventricular dysfunction after heart transplantation: incidence and role of enhanced immunosuppression. J Heart Lung Transplant 1996;15:506-15. [PubMed]
- Sagar KB, Hastillo A, Wolfgang TC, et al. Left ventricular mass by M-mode echocardiography in cardiac transplant patients with acute rejection. Circulation 1981;64:II217-20. [PubMed]
- Garrido IP, Pascual-Figal DA, Nicolás F, et al. Usefulness of serial monitoring of B-type natriuretic peptide for the detection of acute rejection after heart transplantation. Am J Cardiol 2009;103:1149-53. [PubMed]
- Damodaran A, Dardas T, Wu AH, et al. Changes in serial B-type natriuretic peptide level independently predict cardiac allograft rejection. J Heart Lung Transplant 2012;31:708-14. [PubMed]
- Kittleson MM, Skojec DV, Wittstein IS, et al. The change in B-type natriuretic peptide levels over time predicts significant rejection in cardiac transplant recipients. J Heart Lung Transplant 2009;28:704-9. [PubMed]
- Dengler TJ, Zimmermann R, Braun K, et al. Elevated serum concentrations of cardiac troponin T in acute allograft rejection after human heart transplantation. J Am Coll Cardiol 1998;32:405-12. [PubMed]
- Hofmann NP, Steuer C, Voss A, et al. Comprehensive bio-imaging using myocardial perfusion reserve index during cardiac magnetic resonance imaging and high-sensitive troponin T for the prediction of outcomes in heart transplant recipients. Am J Transplant 2014;14:2607-16. [PubMed]
- Sutherland GR, Di Salvo G, Claus P, et al. Strain and strain rate imaging: a new clinical approach to quantifying regional myocardial function. J Am Soc Echocardiogr 2004;17:788-802. [PubMed]
- Korosoglou G, Osman NF, Dengler TJ, et al. Strain-encoded cardiac magnetic resonance for the evaluation of chronic allograft vasculopathy in transplant recipients. Am J Transplant 2009;9:2587-96. [PubMed]