Clinical outcomes of cyberknife stereotactic radiosurgery for lung metastases
Introduction
Metastatic disease is a leading cause of cancer mortality, and the lungs are a common site for metastatic seeding. Surgery is the standard treatment option, with good results in terms of local control (LC) and survival. The studies by the International Registry of Lung Metastases (1) demonstrate that metastasectomy of lung metastases in selected patients can result in long-term survival. The 5-year survival after complete metastasectomy is 36%; however, that for incomplete resection is only 13%. Multivariate analysis showed better prognosis for patients with longer disease-free intervals and single metastases. In addition, complete surgical removal of pulmonary metastases improves survival, as shown in a recent review by Kaifi et al. (2). In 1988, Takegawa (3) reported the actuarial survival after radiation therapy is 27% at 5 years (median, 10.9 months), and the corresponding value for no-treatment is 0% at five years (median, 3.8 months). The present study has confirmed the benefits of radiation therapy for metastases. Technological advances in radiation therapy have enabled stereotactic body radiation therapy (SBRT) as a novel technique for pulmonary tumors. Cyberknife is a stereotactic radiosurgery system that employs real-time image guidance and synchronized respiratory tracking system to deliver high doses of hypofractionated radiation dynamically to the tumor (4). Several groups reported their findings on SBRT in patients with lung metastases (5-7). The 2-year LC values range from 67% to 96%, as shown in a review by Siva et al. (8). Stereotactic radiotherapy has emerged as a viable, effective and well-tolerated alternative to surgery with comparable LC rates (9,10).
In this study, we evaluate the feasibility, safety, and effectiveness of cyberknife for lung metastases based on the results of patients with lung metastases.
Materials and methods
Patients
We performed a retrospective analysis of 95 patients with 134 metastases who were treated with SBRT at our institution from March 2009 to March 2013. Prior to treatment, all patients underwent pertinent studies, including the head magnetic resonance imaging, chest and abdominal computed tomography (CT), routine blood tests, blood chemistry panel, and tumor markers. The patients’ conditions were comprehensively assessed by radiologists and oncologists. This study has been approved by ethics committee board. All patients signed an informed consent for the Cyberknife treatment.
Treatment
Treatments were performed with SBRT using cyberknife (Accuray; Sunnyvale, CA, USA) technology, which was previously described by our group (11). A total of 52 patients who were ineligible for the “X sight lung” option had been implanted with one to three gold fiducials inside or near the tumor to define the tumor position. At approximately 1 week after fiducial placement, CT simulation was performed for treatment planning. Gross tumor volume (GTV) was defined as the tumor volume delineated on lung window settings. The planning target volume (PTV) was obtained by expanding the GTV by 3 mm in all directions. The dose was prescribed based on the isodose line and covered the PTV (generally the 80% to 90% isodose line). Hypofractionated SBRT was delivered at a total dose of 30 to 60 Gy over 1 to 5 d. Dose and fractionation schedules were developed based on the patient’s performance status, tumor size, and location.
Follow-up and statistics
The primary end point of this clinical study was LC; secondary end points were toxicity, progression-free survival (PFS), overall survival (OS), and cancer-specific survival (CSS). All patients underwent clinical examination and CT scan to evaluate the treatment results 4 to 6 weeks after SBRT, followed by CT scans every 3 months until death. Treatment responses were assessed based on the Response Evaluation Criteria in Solid Tumors. New or progressive lesions that form within or at the margin of the PTV were scored as local progression, whereas lesions form outside the PTV were scored as distant progression. Toxicity was assessed according to the National Cancer Institute Common Toxicity Criteria (V3.0) and the Radiation Therapy Oncology Group late toxicity index.
OS was assessed from the start of the cyberknife until death, censoring the last follow-up date. LC rate was calculated from the date of the SBRT to the first local progression (new or progressive lesions arising within or at the margin of the PTV were scored as local progression) date, censoring death or last follow-up date. All patients who started the treatment were included in the analysis. Statistical analyses were performed with paired t-test or χ2 test, as appropriate. The significance was defined at a two-sided P value of <0.05. OS was expressed using Kaplan-Meier survival curves. A log-rank test was used to test for differences in survival rates. LC and PFS was analyzed the same way as OS. Potential prognostic factors tested by univariate and multivariate analysis. Statistical analysis was performed using the SPSS software, version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Patients
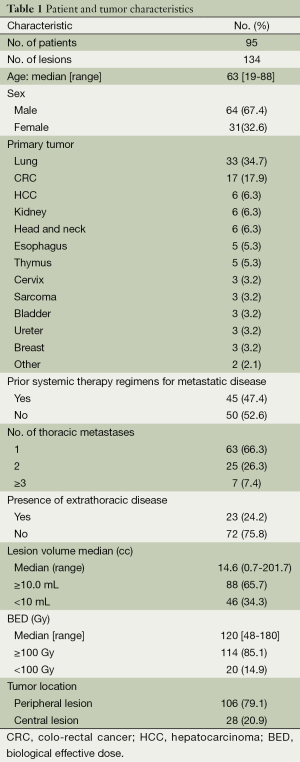
From March 2009 to June 2013, 95 patients with 134 lung metastases were treated at our institution. All patients were assessed for LC and survival. The median follow-up was 17 months (ranging from 4 to 46 months). Patient and tumor characteristics are presented in Table 1. A total of 64 men and 31 women with a median age of 63 years (ranging from 19 to 88 years) were included in the study. The primary tumor was lung (34.7%) and colo-rectal cancer (CRC) (17.9%). Thirty-four patients (35.8%) received chemotherapy after SBRT at the time of systemic progression. A synchronized breathing tracking technique was used in 52 patients (54.7%) with gold fiducial implantation. Nine patients (17.3%) with implanted lung fiducial markers experienced at least a small pneumothorax, and one patient (1.9%) required thoracostomy tubes.
Full table
Local control (LC)
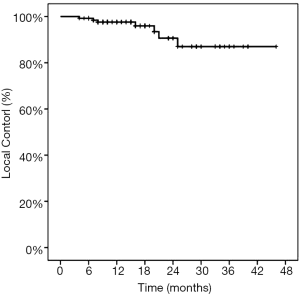
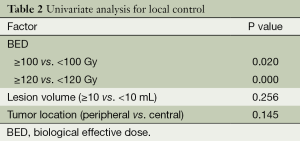
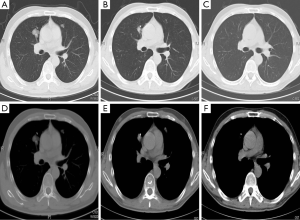
The 1-yearactuarial LC rate was 97.6%, the 2-yearactuarial LC rate was 90.6%, and the 3-yearactuarial LC rate was 87.0%. Seven local treatment failures were recorded. Actuarial LC of the lung lesions is shown in Figure 1. The biological effective dose (BED) was significantly correlated with LC. Based on subgroup analysis, the LC rates of patients who received BED ≥100 Gy were higher than those receiving <100 Gy (P=0.020) (Table 2). The longest duration of LC was in a patient with 46 months of radiographic follow-up after Cyberknife to a single lung lesion (Figure 2).
Full table
Survival
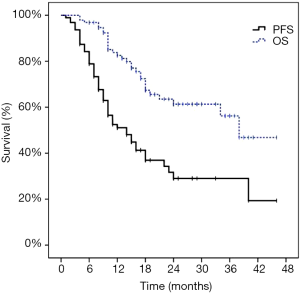
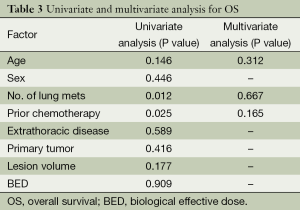
Distant progression occurred in 55 patients (57.9%). The first progression was distant in 53 patients and local in two patients. At the time of analysis, 25 of the 95 patients (26.3%) died of disease progression. The 1-year PFS was 51.1% and the 2-year PFS was 29.0%. The median survival time was 38.0 months and the median PFS time was 14.0 months. The 1-year OS rate was 82.5%, the 2-year OS rate was 61.3%, and the 3-year OS rate was 56.2%. The Kaplan-Meier PFS and OS curves are shown in Figure 3. The univariate and multivariate analysis for OS as shown in Table 3.
Full table
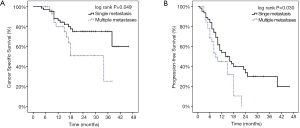
The number of thoracic metastatic lesions was significantly correlated with CSS and PFS. The 2-year CSS was 75.2% for one metastasis, but 51.0% for those with more than one metastasis after SBRT. The patients with one metastasis had a median PFS of 14 months and a 2-year PFS of 33.4%. By contrast, patients with more than one metastasis had a median PFS of 9 months and a 2-year PFS of 0%. The log-rank tests performed on Kaplan-Meier survival estimates confirmed these findings, as shown in Figure 4.
Toxicity
No cases of grade 4 to 5 toxicity or possible treatment-related death were observed. The most common acute toxicity from SBRT was grade 1 fatigue (28/95, 29.5%) and asymptomatic pneumonitis (24/95, 25.3%).Brisk erythema and tenderness of the skin (grade 2 radiation dermatitis) occurred in one patient (1/95, 1.1%). Grade 3 radiation pneumonitis was observed in three patients (3/95, 3.2%) within 2 to 6 months after SBRT, but were discharged after symptomatic treatment.
Discussion
In this study, we have reported outcomes for 95 patients treated with cyberknife for lung metastases. With 134 lesions included in the analysis, the 1-year LC rate was 97.6%, the 2-year LC rate was 90.6%, and 2-year LC rate was 87%. The Cyberknife treatment was well tolerated. Grade 3 radiation pneumonitis only occurred in 3.2% of patients.
A prospective, multi-institutional phase I/II trial involving 38 patients with 61 lung metastases reported a 2-year LC rate of 96%, grade 3 toxicity only occurring in 8% of patients. They also observed a dose-response relationship, with improved LC rates at higher doses (6). Stinauer et al. (12) found that BED (<100 vs. >100 Gy, P<0.01) is a significant predictor of LC. In the current study, 7 of the 134 tumors had local failure. Five of these seven patients (71.4%) had low irradiation dosage (BED <100 Gy), and four patients (57.1%) presented with central lesions. Our subgroup analyses also showed superior LC rates in patients with BED ≥100 Gy (P=0.020), which suggests that the LC rate of cyberknife is associated with the BED. Other studies have indicated that the tumor size of lung metastases is also predictive of LC. Kim et al. (13) reported that tumors <2.5 cm have higher crude local tumor control rates than tumors ≥2.5 cm (100.0% vs. 82.3%, P=0.05). Similarly, Osti et al. treated 66 patients with 103 lung metastases using single-fraction SBRT to a total doses of 23 and 30 Gy and showed a significant correlation between tumor small volume (<10 cc) and LC probability (P<0.024) (14).
The survival results of the current study are globally comparable to those of published series. A review by Siva et al. showed 2-year weighted OS rates ranging from 39% to 84% (8). Rusthoven et al. (6) reported the results of a phase I/II trial using SBRT at a dose of 48 to 60 Gy in three fractions in the treatment of lung metastases with cumulative lesion diameters smaller than 7 cm. The median OS was 19.0 months, and the 2-year OS was 39%. Ricardi et al. reported the results of 61 patients with oligometastatic lung tumors treated with SBRT. After a median follow-up interval of 20.4 months, the 2-year OS was 66.5%. The researchers suggested that tumor volume is significantly associated with survival, with the highest rates occurring in patients with single small tumors (7). Salama et al. showed that the PFS and OS of patients treated for oligometastatic disease are associated with the number of metastases, with patients with one to three metastases exhibiting better survival than those with four to five metastases (15). In the current study, the 2-year PFS and CSS rates of patients with single metastases were higher than those with multiple metastases (33.4% vs. 0%; 75.2% vs. 51.0%, P<0.05).
We observed grade 3 radiation pneumonitis occurring in three patients (3.2%). The V20 of these three patients are 19%, 22%, and 25%. In a prospective phase I/II study (6), the rate of grade 3 radiation pneumonitis was 2.6%,which suggests that the dose constraint used (V15 <35%) was reasonable. McGarry et al. reported an increased incidence of grade 3 and above toxicities when treating early-stage NSCLC patients with target diameters of >5 cm (16). In our study, two of the three patients (66.7%) who developed grade 3 radiation pneumonitis had a cumulative lesion diameter of >5 cm, which suggests that small metastatic tumors (<5 cm) may be reduced in radiation pneumonitis.
Conclusions
In conclusion, the results of the present study and of previous trials support the efficacy and safety of Cyberknife in patients with lung metastases. Highest LC rates can be achieved with higher radiation dose delivered.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [PubMed]
- Kaifi JT, Gusani NJ, Deshaies I, et al. Indications and approach to surgical resection of lung metastases. J Surg Oncol 2010;102:187-95. [PubMed]
- Takegawa Y. Radiation therapy of lung metastases. Gan No Rinsho 1988;34:1121-9. [PubMed]
- Nuyttens JJ, van de Pol M. The CyberKnife radiosurgery system for lung cancer. Expert Rev Med Devices 2012;9:465-75. [PubMed]
- Brown WT, Wu X, Fowler JF, et al. Lung metastases treated by CyberKnife image-guided robotic stereotactic radiosurgery at 41 months. South Med J 2008;101:376-82. [PubMed]
- Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009;27:1579-84. [PubMed]
- Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for lung metastases. Lung Cancer 2012;75:77-81. [PubMed]
- Siva S, MacManus M, Ball D. Stereotactic radiotherapy for pulmonary oligometastases: a systematic review. J Thorac Oncol 2010;5:1091-9. [PubMed]
- Hatime M, Elmorabit B, Elkhoti Y, et al. Oligometastatic disease, a new concept: stereotactic irradiation for lung metastases. Literature review. Cancer Radiother 2012;16:351-7. [PubMed]
- Alongi F, Arcangeli S, Filippi AR, et al. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist 2012;17:1100-7. [PubMed]
- Wang Z, Zhu XX, Wu XH, et al. Gefitinib combined with stereotactic radiosurgery in previously treated patients with advanced non-small cell lung cancer. Am J Clin Oncol 2014;37:148-53. [PubMed]
- Stinauer MA, Kavanagh BD, Schefter TE, et al. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: impact of single fraction equivalent dose on local control. Radiat Oncol 2011;6:34. [PubMed]
- Kim H, Ahn YC, Park HC, et al. Results and prognostic factors of hypofractionated stereotactic radiation therapy for primary or metastatic lung cancer. J Thorac Oncol 2010;5:526-32. [PubMed]
- Osti MF, Carnevale A, Valeriani M, et al. Clinical outcomes of single dose stereotactic radiotherapy for lung metastases. Clin Lung Cancer 2013;14:699-703. [PubMed]
- Salama JK, Hasselle MD, Chmura SJ, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer 2012;118:2962-70. [PubMed]
- McGarry RC, Papiez L, Williams M, et al. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: phase I study. Int J Radiat Oncol Biol Phys 2005;63:1010-5. [PubMed]