Reconstruction techniques for hypopharyngeal and cervical esophageal carcinoma
Introduction
The digestive tract reconstruction techniques commonly used for patients treated surgically for hypopharyngeal and cervical esophageal carcinoma include a tube-shaped skin flap, stomach replacement of the esophagus, or colonic replacement of the esophagus, techniques that connect the esophagus with the pharynx and therefore allow for the reconstruction of the upper digestive tract. However, previous studies have demonstrated that these techniques are associated with a relatively high incidence of perioperative complications. Therefore, we developed a novel surgical method, non-thoracotomic tube stomach replacement of the esophagus, in order to reduce the incidence of perioperative complications. In the present study, the incidence of perioperative complications was compared among 105 patients with hypopharyngeal and cervical esophageal carcinoma who underwent a pectoral major muscle skin flap reconstruction, a stomach replacement of the esophagus, or a tube stomach replacement of the esophagus.
Patients and methods
Patients
Data from 105 patients (87 males and 18 females) with hypopharyngeal and cervical esophageal carcinoma who were treated at SUN YAT-SEN Memorial Hospital from January 2003 to February 2013 were retrospectively analyzed. The mean age of these patients was 51 years (range, 39-71 years). Based on the TNM staging criteria (UICC 1997), the patients were classified as follows: T2N0M0, for ten patients; T2N1M0, for eight patients; T3N1M0, for 25 patients; T3N0M0, for 27 patients; T4N0M0, for 23 patients; and T4N1M0, for 12 patients. Eight patients had hypertension; two patients had coronary heart disease, and two patients had diabetes. All of the patients were diagnosed based on both preoperative imaging studies and pathological examinations. With an average length of 1.4-4.2 cm, the lesions were 16-20 cm away from the incisor. The lesions were always located at the cervical esophagus and invaded the hypopharynx, above, and the sternoclavicular joint, below, to a distance less than 2 cm. Based on the preoperative pathological examinations, all of the patients had esophageal squamous-cell carcinoma (ESCC) (29 patients with highly differentiated carcinoma, 56 patients with moderately differentiated carcinoma, and 20 patients with poorly-differentiated ESCC). No significant differences in age, sex, tumor differentiation, and TNM stage were observed among the three groups (P>0.05).
Reconstruction method
The patients were divided into three groups (groups A, B, and C) based on the reconstruction technique utilized. For the patients in group A (n=45), a pectoral major muscle skin flap reconstruction was performed. Briefly, following the complete resection of the tumor and the necessary adjacent normal tissue, a pedicel flap was used to form a tube; the upper portion of the tube was subsequently anastomosed to the pharynx, and the lower portion was anastomosed to the distal aspect of the cervical esophagus. For the patients in group B (n=32), a stomach replacement of the esophagus was performed. Briefly, an incision was made within the upper left quadrant of the abdomen; the stomach was isolated until the pylorus was reached; the right gastroepiploic artery and vein were preserved; the cervical esophagus was subsequently isolated, and a bougie was inserted from the cervical esophagus into the abdominal esophagus. An esophagectomy was then performed, and the upper end of the stomach was pulled upward and anastomosed to the incisal edge at the pharynx. For the patients in group C (n=28), a tube stomach replacement of the esophagus was performed. Briefly, the entire stomach was isolated, and a GIA-80 stapler (US Surgical, USA) was used to harvest both the cardia and the tissues at the lesser curvature in order to form a tube stomach with a diameter of approximately 3 cm, which was followed by the same procedure as that described regarding the patients in group B.
Follow-up
The patients were followed via out-patient visits, mail, or phone calls.
Statistical analysis
SPSS 16.0 was used for the statistical analysis. The overall survival (OS) rate was calculated using the Kaplan-Meier method from the data collected on the day of the surgery and on the day of the last follow-up visit (October 2014 or the death of the patient). The difference in survival between the two groups was compared using the log-rank test. Comparisons of the other parameters were performed using the Chi-square test. P<0.05 was considered statistically significant.
Results
Following surgery, seven patients developed pulmonary infections; 23 patients developed arrhythmias; five patients developed thoracic stomach syndrome; 28 patients developed esophageal reflux; 25 patients developed an incision infection; 20 patients developed neck swelling, and 29 patients developed anastomotic leakage. Four patients were either discharged against medical advice or died, whereas the remaining patients were treated with dressings and anti-infection agents, and discharged following recovery. The overall incidences of postoperative complications among the three groups were significantly different (P<0.05).
Additional analyses were performed in order to compare the incidences of complications between two groups.
Compared with group A, the incidences of incision infection, neck swelling, and anastomotic leakage were significantly lower in group C (P<0.05), whereas the incidences of other complications were not significantly different between the two groups (P>0.05). Compared with group B, the incidences of arrhythmia, thoracic stomach syndrome, esophageal reflux, and anastomotic leakage were significantly lower in group C (P<0.05), whereas the incidences of other complications were not significantly different between the two groups (P>0.05) (Table 1).
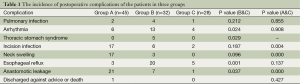
Full table
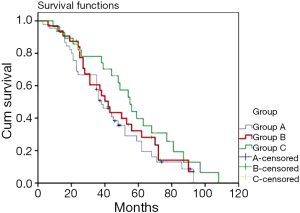
A total of 82.2% (37/45), 78.1% (25/32), and 78.6% (22/28) of the patients in groups A, B, and C, respectively, were successfully followed. The follow-up period ranged from 3 to 108 months. The 1-, 3-, and 5-year survival rates were 93.3%±3.1%, 50.8%±7.5%, and 25.9%±7.2% in group A, 93.8%±4.3%, 57.1%±9.0%, and 28.2%±8.6% in group B, and 92.9%±4.9%, 74.2%±8.4%, and 34.7%±10.0% in group C, respectively. The median survival times were 36, 40, and 55 months in groups A, B, and C, respectively. The difference was statistically significant between groups A and C (P<0.05), but not between either groups B and C or groups A and B (P>0.05) (Figure 1).
Discussion
The cervical esophagus extends from the posterior hypopharyngeal circumference to the suprasternal notch and is approximately 4.5-6 cm long. The pharyngeal and cervical esophagus is a continuous anatomical tissue that connects the upper and lower digestive tract; therefore, cervical esophageal carcinoma generally invades the pharynx (1). As the anatomical structure of the neck is very complex, the most frequently used surgical technique has historically been local tumor resection plus laryngectomy, which is easy to perform, as it requires only an incision in the neck but not in either the thorax or the abdomen. However, this procedure demonstrated only limited effectiveness where the cardio-pulmonary function of the patients who underwent surgery was concerned. The current technique utilized for defect reconstruction following surgery entails the use of a skin flap in order to form a tube, which is implanted into a subcutaneous tunnel and anastomosed to both the pharynx and the distal esophagus in order to replace the resected cervical esophagus. However, reconstruction with a pedicel flap is associated with a relatively high risk of neck swelling and is a difficult procedure, as forming a tube-shape is difficult. Therefore, the length of the tube is relatively short, and the range of reconstruction is limited. Additionally, the tube is covered with skin instead of being allowed to regenerate physiologically, which may result in relatively poor postoperative swallowing. The poor blood flow to the skin flap may also result in a relatively high risk of flap necrosis, a slow recovery of the anastomosis, and a high incidence of anastomotic fistula and stenosis. In the present study, anastomotic leakage, incision infection, and neck swelling were observed in 21 (46.7%), 17 (37.8%) and 17 (37.8%) patients in group A, respectively. Wang et al. (2) also demonstrated that the incidence of anastomotic leakage was relatively high among the patients who underwent a pectoral major muscle skin flap reconstruction.
Several advantages of using the stomach, the most commonly used alternative for replacement of the esophagus following surgery for esophageal carcinoma, have been reported. Excessive neck swelling does not occur following surgery. Additionally, the anatomical structure of the reconstructed tissues is very similar to that of the normal esophagus, which therefore results in a faster recovery, early oral feeding, a low risk of anastomotic leakage and almost-normal postoperative swallowing. The stomach may be isolated as much as approximately 30-33 cm and therefore may be used for the reconstruction of either high defects or relatively large defects. The blood supply to the isolated stomach is provided by the right gastroepiploic artery, which is richer than a pedicel flap; therefore, the incidence of complications, including anastomotic leakage, is much lower. In the present study, the incidences of anastomotic leakage (n=7, 21.9%) and neck swelling (n=3, 9.4%) were significantly lower compared with group A; however, the incidences of arrhythmia, thoracic stomach syndrome, sour regurgitation, and esophageal reflux were significantly higher compared with group A. The significant increase in the incidence of arrhythmia (40.6%) may have been caused by surgical factors (such as a diaphragm incision) and by thoracic gastric dilatation induced by delayed gastric emptying, which may have compressed the heart and the pulmonary tissue, affecting the circulation and inducing arrhythmia (3,4). Our findings were similar to the findings of Qi et al. (5). Sour regurgitation and esophageal reflux are common long-term complications (possibly life-long) of esophagectomy and may severely impact patients’ quality of life. According to previous studies (6-9), 60-80% of the patients who underwent surgery for esophageal carcinoma developed gastroesophageal reflux symptoms, and 27-35% of these patients suffered from reflux esophagitis; some of the patients developed columnar metaplasia (including metaplasia of the intestinal epithelium, Barrett’s esophagus). Several structures, including the lower esophageal sphincter (LES), the His angle (a sharp angle between the esophagus and the fundus of stomach), the phreno-esophageal ligament, and the crura of diaphragm are involved in the prevention of esophageal reflux. However, these structures are often severely damaged as a result of the resection of esophageal carcinoma. Additionally, the vagal nerve trunk is also resected, which inhibits the physiological peristalsis of thoracic stomach; negative pressure in thoracic cavity also reduces the pressure gradient between the stomach and the duodenum (10). The resection of the vagal nerve trunk reduces the peristalsis of stomach antrum, increases the pressure at the pylorus, and decreases the secretion of gastrin. The ectopic pacemaker at the stomach antrum also induces tachygastria and influences normal peristalsis. The combination of each of these factors finally induces delayed gastric emptying (11). Delayed gastric emptying and esophageal reflux may complement each other, which may further aggravate sour regurgitation, reflux, and thoracic gastric dilatation.
The incidence of esophageal reflux was significantly lower in group C than in group B (P<0.05), and the mechanism underlying this finding may be as follows: (I) stomach tissue at the lesser curvature was resected, which directly reduced the number of oxyntic cells and therefore reduced the secretion of gastric acid; (II) the diameter of the tube stomach is similar to that of the esophagus, which may meet the physiological requirements of the reconstructed digestive tract, accelerate the emptying of the thoracic stomach, and decrease the retention time of both food and gastric juice within the thoracic stomach. Zhou et al. (12) observed that both the anal resting pressure and the systolic pressure of the thoracic stomach in the tube stomach group were significantly higher than in the total stomach group, whereas the level of peristalsis was not significantly different between the two groups. However, the emptying of the thoracic stomach is caused primarily by the pressure induced by its contraction, suggesting that receptive relaxation reflex may have been induced easily among the patients of the tube stomach group while swallowing food, which may in turn have resulted in stronger peristaltic systolic pressure and accelerated thoracic stomach emptying. In the present study, we also noted that the incidences of both thoracic stomach syndrome and anastomotic leakage were significantly lower in group C than in group B (P<0.05). Based on previous findings (13,14), the resection of the cardia of the stomach and the tissues at the lesser curvature, as well as accelerated stomach emptying, substantially reduced the dilation of the gastric body and the compression of the heart and lungs, which aided both expectoration and lung functional recovery, which reduced the risk of thoracic stomach syndrome.
Anastomotic leakage following surgery for esophageal carcinoma is caused primarily by high tension and poor blood supply to the anastomosis. Therefore, we hypothesized that the anastomotic leakage and gastric wall necrosis observed in the present study may have been due to the anastomosis’ location at the stomach fundus, near the lesser curvature. As the blood supply to the isolated stomach is provided by the right gastroepiploic artery, the long path for the blood supply and the high position of anastomosis may have resulted in relatively poor blood supply to the anastomosis. The length of the isolated stomach was limited, which may not have completely compensated for the high tension of the anastomosis among patients with high defects; diaphragm rupture and the thoracic inlet may also have compressed the blood vessels within gastric walls, which may have resulted in poor blood supply to the anastomosis and subsequently induced anastomotic leakage (15). Ikeda et al. (16) observed that using a tube stomach effectively increased both the length of the gastric body and the blood supply to the anastomosis, reducing the incidence of anastomotic leakage. Previous studies have demonstrated that using a tube stomach increased the length of the gastric body by 8 cm, which resulted in a longitudinal axis length of 38.5±3.2 cm (17); therefore, an anastomosis may be performed in patients with either high defects or large defects, without any tension. The reduction of the tension of the tube stomach may also reduce the tension on the right gastroepiploic artery and vein and increase the blood supply to the anastomosis. An anastomosis may also be performed at other positions if the tube stomach is long enough. Based on the normal blood supply to the stomach, blood supply is richer for lower anastomoses. Additionally, the cardia and the tissues at the lesser curvature were resected during the tube stomach surgery, which improved the blood supply to the tube stomach and increased the blood supply to the anastomosis by approximately 41% (18). The low tension of the anastomosis also increased the diameters of the blood vessels in the stomach, which improved both arterial perfusion and venous return, and reduced the incidence of anastomotic leakage.
The survival rate was significantly higher in group C than in group A, which may have occurred because no mediastinal lymph node dissection was required when the pedicel flap was used for the repair, as opposed to the techniques used in group C, whereas the residual lymph nodes with ESCC metastasis may have induced relapse and additional metastasis, reducing the survival times of the patients in group A. Additionally, the incidence of anastomotic fistula was higher in group A, which also affected the survival of the patients. PET-CT scanning should be performed in patients with hypopharyngeal and cervical esophageal cancer in order to obtain additional information. Therefore, experienced thoracic surgeons and otorhinolaryngologists should cooperate in performing a total laryngectomy and tube stomach replacement of the esophagus, and also comprehensively evaluate and analyze the diseases affecting their patients in order determine the best surgical method for each patient.
The findings of the present study indicated that tube stomach replacement of the esophagus effectively improved survival and decreased the incidence of postoperative complications. However, the sample size of the present study was relatively small; therefore, the advantages of reducing postoperative pulmonary complications were not well demonstrated. Ongoing related studies may provide additional evidence regarding the advantages offered by this surgical technique.
Acknowledgements
Funding: The work was supported by the state natural sciences fund (Grant No. 81372567).
Disclosure: The authors declare no conflict of interest.
References
- Lei D, Pan X, Xu F, et al. The treatment of hypopharyngeal carcinoma with the involvement of cervical esophagus. Chin J Otorhinolaryngol Head Neck Surg 2005;40:691-5.
- Wang S, Yang X, Zeng Y, et al. Selection of the reconstruction methods of cervical esophagus and hypopharynx. Journal of Central South University 2007;32:524-6.
- Wu C, Chen S, Lin X, et al. Tubular stomach on respiratory function in patients undergoing anastomosis above the aortic arches in the esophageal bed. Chin J Gastrointest Surg 2010;13:450451.
- Guo W, Jiang Y, Wang R, et al. Study on the respiratory function in patietns undergoing different operation approaches of radical surgery for esophageal carcinoma. J Regional Anat & Operative Surg 2009;18:96-8.
- Qi Z, Zhu D, Chen W, et al. The influence of intrathoracic stomach on the respiratory function during the perioperatiove period of esophageal carcinoma. Chin J Thorac Cardiovasc Surg 2000;16:150-2.
- Dresner SM, Griffin SM, Wayman J, et al. Human model of duodenogastro-oesophageal reflux in the development of Barrett's metaplasia. Br J Surg 2003;90:1120-8. [PubMed]
- Yamamoto S, Makuuchi H, Shimada H, et al. Clinical analysis of reflux esophagitis following esophagectomy with gastric tube reconstruction. J Gastroenterol 2007;42:342-5. [PubMed]
- Aly A, Jamieson GG. Reflux after oesophagectomy. Br J Surg 2004;91:137-41. [PubMed]
- Shibuya S, Fukudo S, Shineha R, et al. High incidence of reflux esophagitis observed by routine endoscopic examination after gastric pull-up esophagectomy. World J Surg 2003;27:580-3. [PubMed]
- Panebianco V, Francioni F, Anzidei M, et al. Magnetic resonance-fluoroscopy as long-term follow-up examination in patients with narrow gastric tube reconstruction after radical esophagectomy. Eur J Cardiothorac Surg 2006;30:663-8. [PubMed]
- Hou P, Wu Q. Comparison of the quality of life in patietnts who underwent tubular stomach and replacement of esophagus with stomach after resection for esophageal carcinoma. Chin J Thorac Cardiovasc Surg 2010;26:260-1.
- Zhou G, Xing Y, Qiao F, et al. The influence of replacement of esophagus with tubular stomach on the gastric kinetics after resection for esophageal carcinoma. J Regional Anat & Operative Surg 2013;22:50-2.
- Chen W, Fu X, Xu C, et al. The influence of tubular stomach on gastroesophageal reflux after resection for esophageal carcinoma. Acta Med Univ Sci Technol Huazhong 2011;40:593-6.
- Xu X, Luo J, Zhang S, et al. The application of tubular stomach in radical surgery for esophageal carcinoma. Journal of Nanjing Medical University 2010;30:1327-9.
- Wang ZH, Chen J, Zhu J. Application of gastric tube in operation on hypopharyngeal and cervical esophageal cancer of the advanced stage. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2010;45:246-8. [PubMed]
- Ikeda Y, Tobari S, Niimi M, et al. Reliable cervical anastomosis through the retrosternal route with stepwise gastric tube. J Thorac Cardiovasc Surg 2003;125:1306-12. [PubMed]
- Zhang C, Li J, Zheng J. Feasible study for construction of gastric tube in esophageal reconstruction. Journal of Henan University of Science and Technology 2005;23:175-9.
- Li X, Wang H, Miao G, et al. The application of construction of gastric tube in surgery for esophageal carcinoma. Journal of Clinical Medicine 2010;30:63-4.