Impact of metformin use on survival in locally-advanced, inoperable non-small cell lung cancer treated with definitive chemoradiation
Introduction
Lung cancer is the most fatal cancer worldwide, responsible for nearly one fifth of all cancer deaths (1-3). Among patients with this illness, at least 80% are afflicted with non-small cell lung cancer (NSCLC), and 65% of those patients present with locally advanced or metastatic disease (4). The management of NSCLC is stage-dependent and may involve surgery, chemotherapy, radiation therapy (RT), or a combination of these modalities (5). In general, for Stage II disease deemed operable patients may undergo surgery, usually with post-operative chemotherapy. Treatment of Stage III disease is controversial, partly due to the heterogeneity of disease pattern in this group. Surgery may be considered for Stage IIIA NSCLC and is often preceded by neoadjuvant chemotherapy or chemoradiation (CRT). Alternatively, for unresectable stage II and III NSCLC, the current standard of care is RT to 60-Gy with concurrent platinum-based doublet chemotherapy (6). The poor 5-year survival rates for Stage II to III disease, ranging from 30% to as low as 5%, imply a need for improvements in therapy.
Recently the oral hypoglycemic agent metformin has been linked to a reduction in the overall risk of cancer incidence and mortality in all sites (7-9). In select cancers, retrospective studies have demonstrated a clinical benefit to concurrent metformin use during cancer treatment. For instance, metformin use has been associated with higher pathologic complete response (pCR) rates in patients with esophageal, rectal, and breast cancers (10-12). Furthermore, improvements in survival have been observed in cancers of the larynx, lung, prostate, ovary and rectum (11,13-16). A retrospective analysis of 99 patients by Tan et al. found that metformin, when taken during chemotherapy, significantly improved overall survival (OS) and progression free survival in diabetic patients with advanced stage NSCLC (17). When combined with RT or CRT, metformin has been shown to improve pCR rates in esophageal cancer and survival rates in prostate, laryngeal, and rectal cancers (10,11,13,14). However metformin use was found to have no effect on survival in patients with oropharyngeal cancer treated with RT, nor on patients with pancreatic, colorectal or prostate cancers treated surgically and/or medically (16,18-20). And no studies to date have examined the effect of concurrent metformin with definitive CRT in patients with NSCLC.
On a molecular level, metformin is thought to possess both direct and indirect activity against cancer. Direct effects are related to activation of AMP-kinase (AMPK) which inhibits mammalian target of rapamycin (mTOR). By inhibition of mTOR, metformin impedes protein synthesis and reduces growth and proliferation in cancer cells (21-23). Indirect anticancer effects of the drug involve reduction of serum insulin levels, a hormone known to have mitogenic effects. Metformin has also exhibited lung cancer-specific activity in vitro via radiosensitization of NSCLC cells and inhibition of NSCLC tumor growth (24). In vivo, Algire et al. found that metformin suppressed the detrimental effects of hyperinsulinemia on lung tumor growth in mice (25). These pre-clinical findings may have valuable implications in the clinic.
In this retrospective analysis we investigated survival outcomes in diabetic patients with NSCLC treated with definitive CRT and previously on concurrent metformin. Given the available literature, we hypothesized that NSCLC patients on metformin during CRT might exhibit survival outcomes superior to those not on metformin. If a survival benefit was in fact found, we expected diabetic patients on metformin to do at least as well as non-diabetic patients, and diabetic patients not on metformin to demonstrate significantly poorer survival in comparison. Focusing on locally advanced NSCLC, we hoped to contribute to improving outcomes in the treatment of this devastating and currently intractable disease.
Methods
This single-institution, Institutional Review Board (IRB)-approved retrospective cohort study included patients with NSCLC who were treated definitively with platinum-based doublet chemotherapy and thoracic RT to 60-66 Gy between 1999 and 2013. Patient privacy was maintained in accordance with Health Insurance Portability and Accountability Act regulations.
Our study centered on patients presenting with locally advanced disease. Nevertheless, the occasional patient with stage I unresectable disease was included if treated definitively with radiation. Individuals with stage IV disease were generally excluded except for a subset of patients with oligometastatic disease limited to a solitary extrathoracic metastasis (26). Considering recent evidence of improved survival with focused treatment of oligometastases, these select patients were also treated with curative intent using the standard of care (27-29).
The primary variables of interest were: (I) a history of type II diabetes and (II) concurrent metformin use during CRT. Patients with diabetes must have been diagnosed with anti-diabetic medications started prior to initial consultation with medical or radiation oncology, and any anti-diabetic medications had to be initiated prior to consultation and treatment of their lung cancer. Diabetic patients who were not on metformin were diet-controlled, treated with other medications, or frankly untreated.
All study participants had an initial consultation that involved a history and physical exam. Histologic or cytologic diagnosis confirming adenocarcinoma, squamous cell carcinoma and/or poorly differentiated NSCLC was required for inclusion. Thoracic CT or PET-CT scans were utilized for clinical staging, and patients were staged according to the AJCC TNM Cancer Staging Manual, 6th edition (30). Patients received definitive CRT over a period of six to seven weeks.
During radiotherapy, each patient was placed in a supine position with arms up to allow accurate reproducibility of the target lesion among treatment sessions. A large rigid pillow or mold was created for each patient. RT was delivered using 3D conformal or intensity-modulated technique. RT was delivered through anteroposterior fields first to 40 Gy in 1.8 or 2 Gy per fraction per day followed by oblique fields to avoid the spinal cord for an additional 20-26 Gy for a total RT dose of typically 60-66 Gy. If patients presented with involved bilateral mediastinal lymph nodes, then IMRT was employed either from the onset of RT or for the boost/off-cord component of their RT. The analytic anisotropic algorithm was employed with tissue inhomogeneity corrections, with 6- or 15-Megavoltage photons used to deliver the RT. The radiation dose for the spinal cord was <50 Gy. The mean lung dose was <20 Gy and V5 <60-70% and V20 <37%.
The typical chemotherapy regimen consisted of IV infusional drug delivery consisting of paclitaxel (45 mg/m2/week) plus carboplatin (AUC =2/week) on days 1, 8, 15, 22, 29, 36, and 43 of RT. RT was delivered after the administration of chemotherapy, with RT beginning on Monday with the exception of national holidays occurring on Monday, in which case, RT would begin on Tuesday.
Follow-up chest CT was performed 6-8 weeks after the completion of CRT and then every 3 months for the first year and every 6 months for 2 years, then yearly thereafter. These CT scans were evaluated by thoracic radiology, and medical and radiation oncology. Recurrence was diagnosed and confirmed with clinical exam and imaging by CT or PET-CT.
Study endpoints and statistical analysis
OS was the primary endpoint in this study. Other endpoints of interest included progression-free survival (PFS), locoregional recurrence-free survival (LRRFS) and distant metastasis-free survival (DMFS). All four parameters were quantified using the difference between the date of NSCLC diagnosis and date of last follow-up and/or recurrence. Survival analysis was done comparing DM + met (diabetics treated with metformin) versus non-DM (non-diabetic) cohorts and also DM + met versus DM − met (diabetics not treated with metformin) cohorts.
The computer software program R (version 2.15.1) was used for all statistical analyses (The R Project for Statistical Computing, http://www.r-project.org/). Survival curves were produced using the Kaplan-Meier method, and cohorts were compared using log-rank testing. Chi-squared or Fisher’s exact tests were employed when appropriate, with a P value of 0.05 or less indicating significance. A P value between 0.05 and 0.10 was considered to represent a trend toward significance. Cox regression was used in univariate analysis of all potential confounders. All variables found to significantly influence OS were included in multivariate analysis.
Results
Patient characteristics
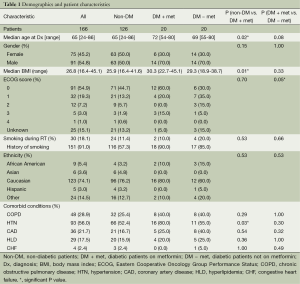
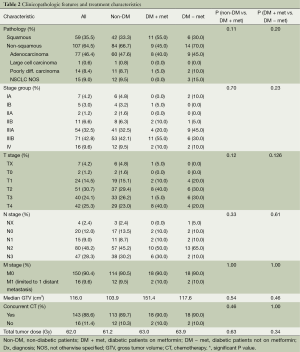
One hundred and sixty-six patients treated with definitive CRT for inoperable NSCLC were eligible for the study (Tables 1 and 2). The median follow-up for the entire study population was 17.0 months. Median age was 65 years, and gender was evenly distributed among cohorts. At 26.8, the median body mass index (BMI) was slightly above the average range (18.5-24.9) and most patients (74.2%) exhibited an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1 prior to treatment (31). Patients of all stages were included, though 83.1% had Stage II or III disease, 7.3% had Stage I disease and 9.6% had Stage IV oligometastatic NSCLC with a single metastasis. T, N and M-stage distributions were similar among cohorts. Among the diabetic patients, the DM + met cohort included three patients on insulin and 11 on other anti-diabetic medications, and the DM-met cohort included six patients on insulin and seven on other antidiabetic medications. Patients’ daily dose of metformin ranged from 500 to 2,000 mg with a median of 2,000 mg. All patients received definitive RT to a median of 62 Gy. Study cohorts consisted of: (I) non-DM, n=126, 76%); (II) DM + met, n=20, 12%; and (III) DM − met, n=20, 12%.
Full table
Full table
Comparative cohort analysis
Non-diabetic patients vs. diabetic patients on metformin
Compared with our non-diabetic cohort, our metformin cohort contained older patients (72 vs. 65 years, P=0.02) with a higher median BMI (30.7 vs. 26.2, P=0.010) and a higher rate of comorbid hypertension (80% vs. 51%, P=0.026). The two cohorts were similar with respect to age, gender, smoking status, ethnicity, and presence of the following comorbidities—chronic obstructive pulmonary disease (COPD), coronary artery disease (CAD), hyperlipidemia (HLD) and congestive heart failure (CHF). Disease characteristics were also similar among cohorts, including tumor pathology, TNM stage, stage group, gross tumor volume (GTV). A similar number of patients in both groups received concurrent chemotherapy, and chemotherapy regimens were similar. Median definitive doses of RT did not differ between cohorts.
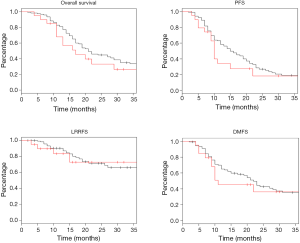
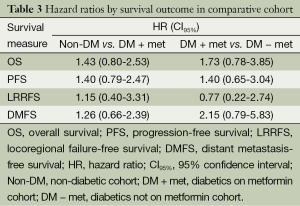
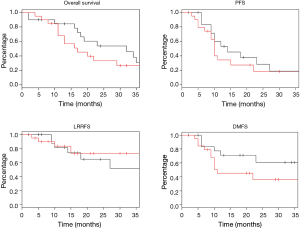
Log rank test comparing non-DM and DM + met patients revealed no significant difference in OS between cohorts (16.3 vs. 14.3 months, P=0.23). PFS was also similar between the two groups with the non-DM group achieving a survival of 11.6 vs. 9.7 months in the DM + met group (P=0.26). Furthermore, concurrent metformin use during CRT seemed to provide no benefit in LRRFS (14.1 vs. 11.9 months, P=0.78) or DMFS (13.4 vs. 10.0 months, P=0.69). Please refer to Figure 1 for Kaplan Meier survival curves and to Table 3 for corresponding hazard ratios with confidence intervals. The 1-, 2- and 3-year OS rates for the non-DM cohort were 69.0%, 30.9% and 19.8% respectively, and were 60.0%, 25.0%, and 10.0% in the DM + met cohort. Identified general negative prognostic factors on univariate analysis included higher age at diagnosis (P=0.03), lower performance status (P=0.005), presence of CAD (P=0.04), higher T stage (P=0.0001), and squamous cell histology (P=0.003).
Full table
Metformin vs. other anti-diabetic therapy in diabetic patients
Diabetic patients on concurrent metformin were compared with diabetic patients who were either concurrently on other medication or diet-controlled. Performance status was significantly worse in DM − met patients when compared with DM + met patients (P=0.05). Otherwise, patient demographics, tumor burden and treatment were similar between the two cohorts (Tables 1 and 2).
Diabetic patients on metformin exhibited comparable survival to diabetic patients on other forms of treatment. Median OS for DM − met patients was 19.2 versus 14.3 months in DM + met patients (P=0.18). PFS in DM − met patients was 10.1 months whereas PFS was 19.7 months for DM + met patients (P=0.38). LRRFS and DMFS were 15.5 and 17.4 months respectively in the DM-met cohort, vs. 11.9 and 10.0 months respectively in the DM + met patients (LRRFS P=0.69, DMFS P=0.12) (Please refer to Figure 2 for Kaplan Meier survival curves and to Table 3 for corresponding hazard ratios with confidence intervals). The 1-, 2- and 3-year OS rates for DM + met cohort were 60.0%, 25.0%, and 10.0% respectively, while they were 80.0%, 35.0% and 20.0% in the DM − met cohort. Non-caucasian ethnicity was the only identified general negative prognostic factor on univariate analysis (P=0.05).
Discussion
We sought to evaluate the relative benefit of metformin on patients being previously treated with this medicine for DM during the course of CRT. Based on literature demonstrating the possible benefit of metformin as an mTOR inhibitor, as well as retrospective clinical studies reporting mixed, site-dependent results with concurrent metformin therapy, we pursued this topic specifically as it applies to patients with locally-advanced unresectable NSCLC patients treated with CRT (10,11,13-16,18-21). In our study, concurrent metformin with CRT did not impact survival or cancer progression. DM patients on metformin exhibited neither a survival benefit when compared with other DM patients, nor when compared with non-DM patients. To our knowledge, this is the first to investigate the effect of concurrent metformin with definitive CRT for locally advanced unresectable NSCLC.
Our study population demonstrated a median OS of 17.0 months and PFS of 10.7 months, with no significant difference among cohorts. It is possible that, due to study limitations, we failed to detect a benefit with metformin use that in fact exists. Compared with the non-DM cohort, the DM + met cohort included significantly older patients with a higher median BMI and a higher rate of comorbid hypertension. Increased age and relatively poorer health may have confounded potentially superior cancer survival in this group. Additionally, due to the retrospective nature of this study, the severity of DM in each diabetic patient was difficult to gauge. Consequently, one weakness of this study is the inability to determine whether the severity of an individual’s DM during CRT for NSCLC impacts outcomes.
The consequences of metformin use may vary by cancer site and patient population. Metformin is contraindicated in patients with renal, hepatic and lung disease due to an increased risk of metabolic acidosis (32). Unfortunately, a majority of NSCLC patients have extensive smoking histories with comorbid obstructive lung disease. In patients using metformin, mortality of reported cases of metabolic acidosis is 50 percent (32). Due to suboptimal ventilation and V/Q mismatch, patients with COPD may suffer from subclinical acidemia, especially with metformin therapy, possibly contributing to relatively poorer survival outcomes in this cohort. This serves as a theoretical possibility in the case of NSCLC, as opposed to that of other cancers not associated with compromised lung function, which have exhibited a survival benefit associated with metformin and concurrent RT (11,13-16).
Additionally, tumor response to metformin may be dose-dependent. A retrospective analysis of 285 patients by Skinner et al. reported a dose-dependent increase in pCR associated with concurrent metformin and CRT in patients with esophageal adenocarcinoma (10). Higher pCR rates were observed in patients taking more than 1,500 mg of metformin daily. Again, as our analysis was retrospectively performed, dosing information was not available for all patients.
Concurrent metformin therapy may only benefit patients with specific tumorigenic mutations. On a molecular level, antineoplastic activity of metformin operates via AMPK and mTOR pathways to inhibit tumor cell growth and proliferation (21). This antineoplastic mechanism may only affect particular classes of malignant cells. Buzzai et al. showed metformin to selectively impair p53 deficient tumor cells in vitro and AICAR—another AMPK activator like metformin—to inhibit p53 -/- tumor growth in vivo (33). If metformin indeed acts specifically on p53 mutated cells, since only 50% of patients with NSCLC possess this mutation, a survival benefit with concurrent metformin might only exist for a subset of patients in our study (34). This difference in survival would be confounded by survival rates of patients in our metformin cohort without a p53 gene mutation.
Also, DM patients in our study population may inherently live longer than patients without DM, potentially masking any benefit conferred by metformin. A Swedish study by Hatlen et al. [2011] retrospectively examined OS in 1,677 lung cancer patients with and without DM. The study reported significantly prolonged OS in lung cancer patients with DM (P=0.005) (35). Although DM is generally associated with poor prognostic outcomes in cancer patients, the relationship between tumor cells, DM and metformin may be unique in lung cancer. The positive results with metformin therapy observed in other cancer sites may not apply to NSCLC.
Conclusions
In summary, our study found no significant survival benefit associated with concurrent metformin during definitive CRT in patients with NSCLC. Metformin may possess differential anti-neoplastic activity depending on patient population, genetics, comorbidities and cancer site. Further retrospective and prospective research is warranted considering positive results in pre-clinical and clinical chemotherapy studies in NSCLC, as well as in clinical studies with other cancer sites. Specifically, analysis of larger populations with more detailed information regarding DM severity, metformin dosing, and tumor genetics is implied.
Acknowledgements
Authors’ contributions: IA contributed to conception of idea and design, collected and analyzed data, drafted the manuscript and performed final revisions. AF performed statistical analysis, assisted in data interpretation and contributed to final revisions of paper. AC, JL, SS, JA and SKJ contributed to data collection and revisions and final approval of the manuscript. In addition, SKJ contributed to conception of idea, study design, data analysis. BGH contributed to study conception and final revisions and approval of manuscript. WZ contributed to data collection, technichal aspects of radiotherapeutic planning and review of technical portions of manuscript.
Disclosure: Dr. Salma K. Jabbour has received an honorarium from Abbott Laboratories. Dr. Joseph Aisner has received ongoing funding from the National Cancer Institute and serves as a board member for Bristol Myers Squibb. The other authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544-73. [PubMed]
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available online: http://globocan.iarc.fr, accessed on 01/03/2014.
- American Cancer Society. Cancer Facts & Figures 2014 [database online]. Atlanta: American Cancer Society, 2014.
- Halperin EC, Perez CA, Brady LW. eds. Perez and Brady’s Principles and Practice of Radiation Oncology. 5th Edition. Lippincott Williams & Wilkins, 2008.
- Reck M, Heigener DF, Mok T, et al. Management of non-small-cell lung cancer: recent developments. Lancet 2013;382:709-19. [PubMed]
- Noto H, Goto A, Tsujimoto T, et al. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One 2012;7:e33411. [PubMed]
- Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA 2008;300:2754-64. [PubMed]
- Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451-61. [PubMed]
- Skinner HD, McCurdy MR, Echeverria AE, et al. Metformin use and improved response to therapy in esophageal adenocarcinoma. Acta Oncol 2013;52:1002-9. [PubMed]
- Skinner HD, Crane CH, Garrett CR, et al. Metformin use and improved response to therapy in rectal cancer. Cancer Med 2013;2:99-107. [PubMed]
- Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 2009;27:3297-302. [PubMed]
- Sandulache VC, Hamblin JS, Skinner HD, et al. Metformin use is associated with improved survival in patients with laryngeal squamous cell carcinoma. Head Neck 2014;36:1039-43. [PubMed]
- Spratt D, Zhang C, Zumsteg ZS, et al. Metformin improves prostate cancer-specific survival and inhibits the development of castrate resistant metastasis. Int J Radiat Oncol Biol Phys 2012;84:S93.
- Romero IL, McCormick A, McEwen KA, et al. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet Gynecol 2012;119:61-7. [PubMed]
- Kaushik D, Karnes RJ, Eisenberg MS, et al. Effect of metformin on prostate cancer outcomes after radical prostatectomy. Urol Oncol 2014;32:43.e1-7.
- Tan BX, Yao WX, Ge J, et al. Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes. Cancer 2011;117:5103-11. [PubMed]
- Hwang AL, Haynes K, Hwang WT, et al. Metformin and survival in pancreatic cancer: a retrospective cohort study. Pancreas 2013;42:1054-9. [PubMed]
- Cossor FI, Adams-Campbell LL, Chlebowski RT, et al. Diabetes, metformin use, and colorectal cancer survival in postmenopausal women. Cancer Epidemiol 2013;37:742-9. [PubMed]
- Patel T, Hruby G, Badani K, et al. Clinical outcomes after radical prostatectomy in diabetic patients treated with metformin. Urology 2010;76:1240-4. [PubMed]
- Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med 2011;9:33. [PubMed]
- Dowling RJ, Zakikhani M, Fantus IG, et al. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res 2007;67:10804-12. [PubMed]
- Gotlieb WH, Saumet J, Beauchamp MC, et al. In vitro metformin anti-neoplastic activity in epithelial ovarian cancer. Gynecol Oncol 2008;110:246-50. [PubMed]
- Storozhuk Y, Hopmans SN, Sanli T, et al. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer 2013;108:2021-32. [PubMed]
- Algire C, Zakikhani M, Blouin MJ, et al. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr Relat Cancer 2008;15:833-9. [PubMed]
- Jabbour SK, Daroui P, Moore D, et al. A novel paradigm in the treatment of oligometastatic non-small cell lung cancer. J Thorac Dis 2011;3:4-9. [PubMed]
- Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol 2011;8:378-82. [PubMed]
- Ashworth A, Rodrigues G, Boldt G, et al. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer 2013;82:197-203. [PubMed]
- Griffioen GH, Toguri D, Dahele M, et al. Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): patient outcomes and prognostic factors. Lung Cancer 2013;82:95-102. [PubMed]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer, 2010:253-70.
- Gahagan S. Overweight and obesity. In: Kliegman RM, Behrman RE, Jenson HB, et al. eds. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Saunders Elsevier, 2011:chap 44.
- Bailey CJ, Turner RC. Metformin. N Engl J Med 1996;334:574-9. [PubMed]
- Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res 2007;67:6745-52. [PubMed]
- Mogi A, Kuwano H. TP53 mutations in nonsmall cell lung cancer. J Biomed Biotechnol 2011;2011:583929.
- Hatlen P, Grønberg BH, Langhammer A, et al. Prolonged survival in patients with lung cancer with diabetes mellitus. J Thorac Oncol 2011;6:1810-7. [PubMed]


