Lung protective effects of budesonide nebulization during perioperative period of thoracolumbar fusion
Introduction
Indications for orthopaedic surgeries are expanding along with the advance of medical sciences and techniques. As China evolves into an aging society, a significant percentage of middle-aged and elderly patients undergo surgery for degenerative thoracolumbar disorders each year. Compared with younger age groups, elder patients usually present with multiple pulmonary and cardiovascular co-morbidities, weakened airway defense, and in males, a long history of cigarette smoking (1,2). Several perioperative factors have been shown to affect the airway and reduce postoperative pulmonary function, resulting in a variety of respiratory complications such as pulmonary atelectasis, pneumonia, bronchospasm, respiratory failure, and acute respiratory distress syndrome (3-6). Pulmonary conditions are among the most common complications following thoracolumbar fusion (5,7), with the perioperative incidence being 5.5% and 7%, respectively, in patients with thoracic and lumbar spinal stenosis. In addition, the incidence of respiratory complication is closely related to the 2-year mortality (relative risk: 10.76) (6). Proactive prevention against pulmonary complications is therefore essential to improve the surgical outcomes and facilitate postoperative recovery in the elderly.
Budesonide is a glucocorticoid typically administered through nebulization. In addition to its powerful anti-inflammatory actions, budesonide may also reduce airway edema, inhibit airway remodeling, and is widely used in treating chronic obstructive pulmonary disease, asthma and many other pulmonary disorders (8-10). During the recent years, perioperative use of budesonide in selected patients for cardiac surgery, thoracic surgery and other procedures has yielded satisfactory pulmonary protective effects (11-15). Pulmonary protection associated with perioperative budesonide nebulization has not been reported in middle-aged or elderly patients undergoing spinal fusion for treatment of thoracolumbar degenerative diseases. We set out to investigate whether budesonide inhaled perioperatively protects against occurrence of pulmonary complications in this subset of patients.
Patients and methods
Patient selection
Between January 2013 and December 2013, forty middle-aged or elderly subjects were randomly selected from all patients undergoing thoracolumbar fusion at the Orthopaedics Department of Peking Union Medical College Hospital. The inclusion criteria were: (I) a confirmed diagnosis of degenerative thoracolumbar disease based on clinical findings and imaging studies; (II) age above 45 years old; (III) risk factors for perioperative pulmonary complications (such as advanced aged >65; BMI ≥28.0; preoperative bedridden time ≥3 days; smoking index ≥400; previous cardiac events; diabetes; cerebrovascular disease; COPD; hypertension; anemia; ASA classification >2; FEV1 ratio ≤65%; operation time ≥3 hours); (IV) with surgical indications for posterior spinal fusion; (V) use of general anesthesia; and (VI) no difficulty in verbal and written communication in Chinese. The exclusion criteria were: (I) use of systemic glucocorticoids within the four weeks prior to surgery; (II) any contraindication for the medication with budesonide, such as drug-related hypersensitivity; (III) necessity of perioperative mechanical ventilation; and (IV) severe co-morbidities of the heart, lung, liver or other vital organs. Those with concomitant trauma of the chest, abdomen or the head, or signs of adrenocortical dysfunction, pregnant or lactating women, and patients who refused to participate in the study were also excluded.
This study was approved by the Ethics Committee of Peking Union Medical College Hospital. All enrolled patients gave informed written consent before entering the study.
Study design and patient treatments
The 40 patients were divided into the control and budesonide groups comprising 20 individuals each using a random number table. All patients underwent general anesthesia with endotracheal intubation before total laminectomy and spinal fusion. All surgeries were performed by the same orthopaedic surgical team. All patients received routine supportive care such as rehydration, analgesia, and neurotrophic drug treatment postoperatively. Antibiotics were discontinued 24 h postoperatively. Non-steroidal anti-inflammatory drugs were administered perioperatively to relieve pain.
In addition, the budesonide group was given 1 mg Pulmicort Respules (budesonide inhalation suspension, AstraZeneca Plc., Sweden) dissolved in 2 mL of normal saline delivered by nebulization (PARI LC Disposable Nebulizer, PARI GmbH., Germany) from days 1 through 3 postoperatively. Instructions to these patients to ensure proper use of budesonide nebulization were given and enhanced before and after the surgical operations. The nebulization lasted 20 min for each session and was performed twice daily (at 9 am and 3 pm).
The investigators of this study were blinded to group assignment; thus, all perioperative data were evaluated in a single-blinded manner.
Data collection
Pulmonary function parameters were measured using spirometry (Master Screen IOS, JAEGER, Germany) 1 day before nebulization: forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and FEV1/FVC. Arterial blood gas analysis was performed when the patients were breathing room air at 1 day before nebulization and at 3 days postoperatively after discontinuing nebulization (4 pm) to estimate pulmonary ventilation and air exchange functions.
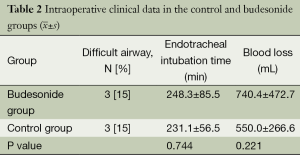
Intraoperatively, presence of a difficult airway (16), endotracheal intubation time, and blood loss were recorded.
Clinical data were observed and recorded daily as follows: (I) general vital signs including body temperature, heart rate, and respiratory rate; (II) pulmonary complications including atelectasis, pneumonia, respiratory failure, bronchospasm, pulmonary embolism, and pulmonary edema, which were diagnosed based on clinical symptoms (coughing, sputum, wheezing, dyspnea), physical checkup, imaging and pathological studies; and (III) nebulization-related adverse reactions: symptoms and clinical signs attributable to glucocorticoids or drug administration (nausea or vomiting) during observation (from the beginning of nebulization to hospital discharge), apart from allergic reactions secondary to other drugs. Adverse reactions were classified into three categories according to severity: mild, with tolerable symptoms or vital signs; moderate, modest interference with normal activities; and severe, noted by severe adverse reactions and inability for normal activities (17).
Statistical analysis
All numerical data were expressed as mean ± standard deviation (SD). Normally distributed data were compared using the independent sample t test, and non-normally distributed data using the rank sum test. Enumeration data were expressed as sample size and percentage, and analyzed using the Chi-square test or Fisher’s exact test. A P value less than 0.05 was considered statistically significant. All data were processed using PASW Statistics for Windows (Version 18.0, SPSS Inc., Chicago, USA).
Results
Patient demographics at baseline
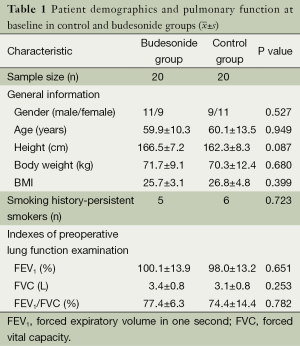
In total, there were 40 patients included in this study, comprising 20 men (50%) and 20 women (50%) aged 46-81 years (mean: 62.4±9.4 years). Before the operations (baseline), there were no statistically significant differences between the two groups with respect to age, height, body weight, BMI, and gender ratio (all P>0.05), as shown in Table 1. Five patients in the budesonide group and six in the control group had a smoking history (P=0.723). The three pulmonary function indices, FEV1, FVC, and FEV1/FVC, were not significantly different between the two groups (all P>0.05), as shown in Table 1.
Full table
Intraoperative data
Three patients (15%) each in the budesonide and control groups had a combined difficult airway due to previous cervical vertebral surgery. The both groups did not differ statistically in intraoperative endotracheal intubation time or blood loss, as shown in Table 2.
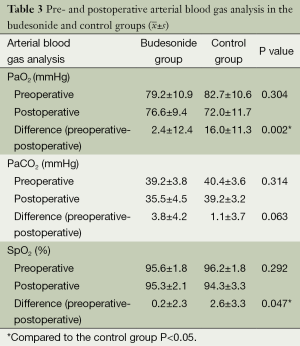
Arterial blood gas
There were no significant differences in the preoperative arterial blood gas analyses (PaO2, PaCO2, SpO2) between the two groups (P>0.05); however, the reduction in levels of postoperative PaO2 and SpO2 from pre-operation was significantly less in the budesonide group than the findings in the control group (P<0.05), as summarized in Table 3.
Full table
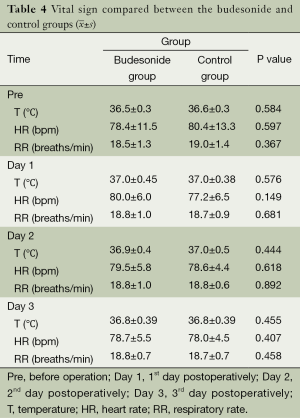
Postoperative vital signs
General vital signs including body temperature, heart rate, and respiratory rate during preoperative days 1 through 3 days did not show significant differences between the groups (P>0.05), as shown in Table 4, suggesting that budesonide nebulization perioperatively did not significantly influence the general postoperative vital signs.
Pulmonary symptoms and complications
No patients in the budesonide group experienced respiratory symptoms such as coughing, expectoration, or wheezing. Three patients (15%) in the control group experienced respiratory symptoms during the study period: two patients (10%) reported coughing and expectoration on the postoperative day 2, and one (5%) reported wheezing and dyspnea on postoperative day 3. All symptoms resolved after symptomatic and supportive treatment, and there were no significant findings in imaging and pathological studies. The budesonide group did not experience any pulmonary complications, compared with one patient in the control group diagnosed with pneumonia based on imaging study and clinical signs including fever, coughing, and expectoration. The patient was given medical treatment and recovered uneventfully.
None patients of the budesonide group experienced events associated with glucocorticoid administration such as allergic reactions, oropharyngeal disease, and systemic reactions. One patient (5%) had a local discomfort related to nebulization, manifesting as nausea during nebulization on the first postoperative day. The patient’s vital signs were stable; the adverse event was deemed attributed to the nebulization procedure, and classified as a mild adverse reaction according to the described classification standards. He recovered with bed-rest and did not require additional treatment.
Discussion
This randomized controlled study indicated that perioperative budesonide nebulization reduced fluctuations in PaO2 and SpO2, and was associated with lower incidence of pulmonary symptoms such as coughing, wheezing, and dyspnea, and pulmonary complications in middle-aged and elderly patients after thoracolumbar fusion. None patients of the budesonide group experienced complications associated with glucocorticoids such as allergic reactions, oropharyngeal disease, and systemic reactions. Moreover, nebulization as administered in this study (1-mg budesonide in 2-mL saline given twice daily from 1 day preoperatively to 3 days postoperatively) was safe and effective for perioperative clinical care.
During thoracolumbar fusion, perioperative pulmonary complications associated with patient age warrant consideration (4,18,19). In degenerative thoracolumbar spinal diseases, a variety of factors can affect occurrence of perioperative pulmonary complications. Preoperative factors include advanced age, cardiopulmonary disease, and a long history of smoking, impaired airway defensive capacity; intraoperative factors such as placement in a prone position, long surgical duration, large surgical incision, endotracheal intubation, anesthetic drugs, and mechanical ventilation, can in many ways stimulate the airway by destroying the respiratory barrier, damaging the tracheal mucosa, and reducing the lung compliance. Postoperative factors such as pain and immobilization limit coughing, prevents discharge of airway secretions. These numerous factors can result in a series of perioperative pulmonary complications such as atelectasis, pneumonia, and bronchospasm. In the 40 middle-aged and elderly patients included in this study, the incidence of pulmonary complications was 2.5% and 5% in the control group. In a clinical study with large sample sizs, He et al. (7) reported a 5.5% perioperative incidence rate of pulmonary complication in thoracic spinal stenosis patients; Lee et al. reported a 7% incidence after lumbar surgery and 13% after spinal surgery (6). The pulmonary complication rate in this study was slightly lower than that reported previously, most probably as a result of rigorous criteria for patient inclusion. In order to control for potential confounders and more objectively evaluate the pulmonary protective effect of budesonide nebulization, patients requiring perioperative mechanical ventilation, concurrent severe or uncontrolled systemic disease such as vital organ failure, concurrent thoracic, abdominal, and head trauma, and those at high risk of severe pulmonary complications were excluded.
Pulmonary protection refers to proactive prevention and treatment of imminent pulmonary injury of various etiologies (20). An appropriate protective strategy during the perioperative period of thoracolumbar fusion can effectively maintain pulmonary function and prevent related complications. This ensures that the patient safely survives the perioperative period and strongly supports the effectiveness of the operation to help patients recover faster.
Budesonide as an inhaled glucocorticoid may effectively inhibit a variety of inflammatory cells, reduces the generation of inflammatory mediators, and exhibits significant anti-inflammatory effect; in the airway, it also induces vessel contraction, inhibits mucosal edema, reduces cell exudation, mitigates edema, and prevents airway remodeling (21-24). Compared to systemic glucocorticoids, such as dexamethasone and hydrocortisone, budesonide nebulization offers the following unique benefits: (I) high concentration primarily in the lungs; (II) high hepatic clearance; and (III) high glucocorticoid receptor affinity. During nebulization, the nebulizer unit breaks the liquid into micro-particles, which are directly inhaled into the lower respiratory tract and rapidly absorbed by the pulmonary mucosa, thus increasing the local drug concentration. It also hydrates the airways, which dilutes airway secretions and facilitates their discharge.
For patients undergoing thoracolumbar fusion, the pharmacologic and pharmacokinetics characteristics of budesonide, combined with local administration using nebulization effectively prevents airway inflammation, alleviates edema, inhibits airway remodeling, and promotes expectoration, which maintains pulmonary function and reduces adverse events associated with glucocorticoid accumulation, such as hypothalamic-pituitary-adrenal axis suppression, bone demineralization, and growth inhibition (25,26). Budesonide nebulization could function as a pulmonary protectant during the perioperative period of thoracolumbar fusion.
This randomized controlled study is the first attempt to evaluate the pulmonary protective effect of budesonide nebulization during perioperative thoracolumbar fusion in middle-aged and elderly patients. Preoperative factors such as age, general health, pulmonary function, and smoking history, which are associated with perioperative pulmonary complications, were consistent between the budesonide and control patients. Reportedly, the presence of a difficult airway affects respiratory function during the perioperative period (16); endotracheal intubation time and the surgical trauma severity also influence the pulmonary complication rate after spinal surgery (6). In this study, the intraoperative blood loss was an indicator for severity of surgical trauma. There were no statistically significant differences intraoperatively between the two groups. Postoperatively, the budesonide group showed remarkably less reduction from baseline in PaO2 and SpO2 as compared with the control group (P<0.05). In addition, incidence of respiratory symptoms such as coughing, asthma, and dyspnea was 15% in the control group, and one (5%) postoperative pneumonia case was observed; the incidences of pulmonary symptoms and complications were higher in the control group than in the budesonide group. These findings indicate that budesonide nebulization perioperatively has a pulmonary protective effect in middle-aged and elderly patients with degenerative thoracolumbar disease, and specifically, nebulization administered as presently described (1-mg budesonide/2-mL saline, twice daily, from 1 day preoperatively to 3 days postoperatively) is clinically effective.
Recently, budesonide has been used more widely perioperatively in several surgical fields including cardiac, thoracic, and abdominal surgeries, and the treatment has achieved good effects (12,14,27-29). Fang et al. (12) evaluated the effect of budesonide nebulization on pulmonary function in a study of 22 elderly patients undergoing abdominal surgery and found that pre- and intraoperative budesonide inhalation improved pulmonary ventilation with no impact on respiratory mechanics. In a randomized controlled study, Ju et al. (14) evaluated the effect of preoperative budesonide nebulization on inflammation in thoracotomy patients receiving single-lung ventilation and revealed that preoperative budesonide nebulization reduced the peak airway pressure and plateau pressure, increased pulmonary compliance, inhibited inflammation, and ultimately improved pulmonary function. A study conducted by Sawada et al. (28) also confirmed the effectiveness of preoperative budesonide nebulization in thoracic surgery patients. Collectively, the results of these prior studies and the current study show that perioperative budesonide nebulization can provide effective pulmonary protection in numerous procedures.
In this study, inhaled corticosteroids in the budesonide group were given in a short duration and low dose. General vital signs such as body temperature, heart rate, and respiratory rate during this period were similar between the groups, and none of the patients experienced allergic reactions, oropharyngeal disease secondary to glucocorticoid nebulization, or systemic adverse reactions; only one patient reported transient nausea following nebulization, which resolved after a short period of rest. These results indicate that in the targeted patient population, short-course perioperative budesonide nebulization as administered in the present study protocol was safe. Presumably, the local administration of budesonide restricted its effect on the lung; this, combined with a higher hepatic clearance, causes fewer systemic adverse reactions compared to intravenous glucocorticoid administration. Previous reports indicate that systemic reactions and oropharyngeal complications caused by budesonide depended on the dosage and treatment duration and occurred solely in patients receiving long-term and large-dosages. Otherwise, adverse reactions were mostly mild, and the complication rate of short-course budesonide treatment was low (8,30,31). Both the present study and previous studies confirm that short-course low-dose budesonide treatment during perioperative thoracolumbar fusion is clinically safe. Budesonide was well tolerated and had a low rate of allergic reactions; however, there are individual reports of delayed allergic reactions following budesonide nebulization (32,33). Although none of the patients experienced an allergic reaction, this complication should be considered in future clinical application of budesonide.
There were several potential limitations in this study. The sample size was small, and patients were not stratified according to factors such as age and smoking history. Correlation factors analysis of pulmonary complications was not performed due to the small sample size. In addition, postoperative follow-up with pulmonary function test was not performed due to potential pain and inconvenience to the patient, and other factors. These limitations may introduce bias in evaluating the clinical effectiveness of budesonide nebulization. Therefore our findings need further validation in future large-sample studies.
In conclusion, perioperative budesonide nebulization may reduce the incidence of pulmonary complications and improved clinical symptoms in middle-aged and elderly patients with thoracolumbar degeneration undergoing spinal fusion. Perioperative budesonide inhalation in this patient population is also tolerable.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Wang Ld. Tobacco or Health? J Transl Intern Med 2014;2:7-10.
- Filippidou EC, Liolios E, Ioannidi V, et al. The smoking habits in rural population and its effects on the lung. J Thorac Dis 2012;4:AB38.
- Schoenfeld AJ, Carey PA, Cleveland AW 3rd, et al. Patient factors, comorbidities, and surgical characteristics that increase mortality and complication risk after spinal arthrodesis: a prognostic study based on 5,887 patients. Spine J 2013;13:1171-9. [PubMed]
- Wider J, Przyklenk K. Ischemic conditioning: the challenge of protecting the diabetic heart. Cardiovasc Diagn Ther 2014;4:383-96. [PubMed]
- Franchitto A, Onori P, Renzi A, et al. Recent advances on the mechanisms regulating cholangiocyte proliferation and the significance of the neuroendocrine regulation of cholangiocyte pathophysiology. Ann Transl Med 2013;1:27. [PubMed]
- Lee MJ, Konodi MA, Cizik AM, et al. Risk factors for medical complication after spine surgery: a multivariate analysis of 1,591 patients. Spine J 2012;12:197-206. [PubMed]
- He B, Yan L, Xu Z, et al. Treatment strategies for the surgical complications of thoracic spinal stenosis: a retrospective analysis of two hundred and eighty three cases. Int Orthop 2014;38:117-22. [PubMed]
- Hernando R, Drobnic ME, Cruz MJ, et al. Budesonide efficacy and safety in patients with bronchiectasis not due to cystic fibrosis. Int J Clin Pharm 2012;34:644-50. [PubMed]
- Jorens PG. Glucocorticoids: Basic in depth. J Transl Intern Med 2014;2:98-9.
- Han D, Wang GZ, Xie XM, et al. Effect and safety of budesonide nebulization on acute and exacerbated chronic obstruction pulmonary disease by Meta analysis. Chin J Intern Med 2013;52:975-7.
- Verborgh C. Corticosteroids in cardiac anesthesia. J Transl Intern Med 2014;2:76-7.
- Fang JY, Huang WQ, Xu KQ, et al. Changes in preoperative and intraoperative abdominal surgeries by budesonide nebulization in elderly patients during the perioperative period. Chin J of Anesthesiol 2005;25:714-5.
- Cheng AB, Shang XJ. Airway protective effect of budesonide suspension for patients undergoing general anesthesia. J Pract Med 2013;29:675-6.
- Ju NY, Gao H, Huang W, et al. Therapeutic effect of inhaled budesonide (Pulmicort® Turbuhaler) on the inflammatory response to one-lung ventilation. Anaesthesia 2014;69:14-23. [PubMed]
- Zhang JP, Zhang RD, Bai J, et al. Lung effect of budesonide nebulization for children with congenital heart disease during the perioperative period. J Clin Res 2011;28:1043-5.
- Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013;118:251-70. [PubMed]
- Pohunek P, Kuna P, Jorup C, et al. Budesonide/formoterol improves lung function compared with budesonide alone in children with asthma. Pediatr Allergy Immunol 2006;17:458-65. [PubMed]
- Cloyd JM, Acosta FL Jr, Cloyd C, et al. Effects of age on perioperative complications of extensive multilevel thoracolumbar spinal fusion surgery. J Neurosurg Spine 2010;12:402-8. [PubMed]
- Kalanithi PS, Patil CG, Boakye M. National complication rates and disposition after posterior lumbar fusion for acquired spondylolisthesis. Spine (Phila Pa 1976) 2009;34:1963-9. [PubMed]
- Wang TY. Expert consensus on lung protection of thoracic surgery during the perioperative period. Chin J Surg 2009;47:1361-4.
- Perng DW, Su KC, Chou KT, et al. Long-acting β2 agonists and corticosteroids restore the reduction of histone deacetylase activity and inhibit H2O2-induced mediator release from alveolar macrophages. Pulm Pharmacol Ther 2012;25:312-8. [PubMed]
- Bos IS, Gosens R, Zuidhof AB, et al. Inhibition of allergen-induced airway remodelling by tiotropium and budesonide: a comparison. Eur Respir J 2007;30:653-61. [PubMed]
- Boorsma M, Lutter R, van de Pol MA, et al. Long-term effects of budesonide on inflammatory status in COPD. COPD 2008;5:97-104. [PubMed]
- Fang Q, Schulte NA, Kim H, et al. Effect of budesonide on fibroblast-mediated collagen gel contraction and degradation. J Inflamm Res 2013;6:25-33. [PubMed]
- Chung KF, Caramori G, Adcock IM. Inhaled corticosteroids as combination therapy with beta-adrenergic agonists in airways disease: present and future. Eur J Clin Pharmacol 2009;65:853-71. [PubMed]
- Fahim A, Faruqi S, Wright CE, et al. Comparison of the effect of high-dose inhaled budesonide and fluticasone on adrenal function in patients with severe chronic obstructive pulmonary disease. Ann Thorac Med 2012;7:140-4. [PubMed]
- Ocalan K, Solak O, Esme H, et al. Efficacy of budesonide and interleukin-10 in an experimental rat model with isolated bilateral pulmonary contusion created by blunt thoracic trauma. Eur J Cardiothorac Surg 2013;43:163-7. [PubMed]
- Sawada S, Suehisa H, Yamashita M. Inhalation of corticosteroid and β-agonist for persistent cough following pulmonary resection. Gen Thorac Cardiovasc Surg 2012;60:285-8. [PubMed]
- Ju YN, Gao H, Huang W, et al. Effects of preoperative budesonide nebulization on inflammatory responses in patients undergoing thoracic surgery during one-lung ventilation. Chin J Anesthesiol 2013;33:714-7.
- Sin DD, Tashkin D, Zhang X, et al. Budesonide and the risk of pneumonia: a meta-analysis of individual patient data. Lancet 2009;374:712-9. [PubMed]
- Melamies M, Vainio O, Spillmann T, et al. Endocrine effects of inhaled budesonide compared with inhaled fluticasone propionate and oral prednisolone in healthy Beagle dogs. Vet J 2012;194:349-53. [PubMed]
- Salava A, Alanko K, Hyry H. A case of systemic allergic dermatitis caused by inhaled budesonide: cross-reactivity in patch tests with the novel inhaled corticosteroid ciclesonide. Contact Dermatitis 2012;67:244-6. [PubMed]
- Baeck M, Pilette C, Drieghe J, et al. Allergic contact dermatitis to inhalation corticosteroids. Eur J Dermatol 2010;20:102-8. [PubMed]